Abstract
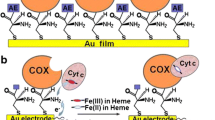
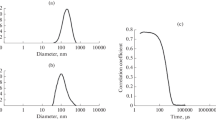
Protein–protein interactions (PPIs) are crucial for the successful realization of many metabolic and signaling pathways. Of particular interest are PPIs of membrane proteins, which form stable and/or transient complexes for the signal transduction, ion transport, and electron transfer within electron transfer chains. The Surface Plasmon Resonance (SPR) allows analyzing the thermodynamic and kinetic parameters (kon and koff) and equilibrium constants (Kd) of PPI, as well as the assessment of the effects of low molecular weight compounds. The aim of the present study was the adaptation of the SPR protocols for PPIs involving mitochondrial cytochrome P450 CYP11A1, mitochondrial cytochrome b5 (CYB5B), and adrenodoxin (Adx). We found that the Adx–CYP11A1 and CYB5B–CYP11A interactions depend on the method of protein immobilization and on the microenvironment. For example, the koff values for complexes Adx–CYP11A1 and CYB5B–CYP11A1 in the aqueous environment were similar (1.5 ± 0.2) × 10–3 s–1, while the values of kon for these complexes differed from each other by almost one order of magnitude ((6.5 ± 0.5) × 104 and (0.30 ± 0.03) × 104 M–1 s–1, respectively). This is in good agreement with the known high affinity of CYP11A1 to its cognate redox partner Adx. In the lipid environment, the rate of complex dissociation was higher than that in the aqueous environment with koff value equal to (9.1 ± 0.3) × 10–3 s–1. For the CYP11A1–CYB5B complex, the parameters of interaction in the lipid phase were not determined due to unspecific binding of the proteins used as analytes to the lipids immobilized on the L1 chip. We show for the first time that SPR method could be used for the detection and quantitative analysis of PPI involving mitochondrial cytochromes (CYP) with their redox partners in both aqueous and lipid environment. Experimental protocols developed and validated in our previous work and in this one can serve as valuable tools for studies of interactions of CYP proteins and could also be applied for other membrane proteins.



Similar content being viewed by others
REFERENCES
Yablokov E.O., Sushko T.A., Ershov P.V., Florinskaya A.V., Gnedenko O.V., Shkel T.V., Grabovec I.P., Strushke-vich N.V., Kaluzhskiy L.A., Usanov S.A., Gilep A.A., Ivanov A.S. 2019. A large-scale comparative analysis of affinity, thermodynamics and functional characteristics of interactions of twelve cytochrome P450 isoforms and their redox partners. Biochimie. 162, 156–166.
Strushkevich N., MacKenzie F., Cherkesova T., Grabovec I., Usanov S., Park H.-W. 2011. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc. Natl. Acad. Sci. USA. 108 (25), 10 139–10 143.
Yablokov E., Florinskaya A., Medvedev A., Sergeev G., Strushkevich N., Luschik A., Shkel T., Haidukevich I., Gilep A., Usanov S., Ivanov A. 2017. Thermodynamics of interactions between mammalian cytochromes P450 and b5. Arch. Biochem. Biophys. 619, 10–15.
Gnedenko O.V., Yablokov E.O., Ershov P.V., Svirid A.V., Shkel T.V., Gaidukevich I.V., Strushkevich N.V., Gilep A.A., Usanov S.A., Ivanov A.S. 2019. Interaction of prostacyclin synthase with cytochromes P450. Biomed. Khimia (Rus.). 65 (1), 63–66.
Guryev O., Carvalho R.A., Usanov S., Gilep A., Estabrook R.W. 2003. A pathway for the metabolism of vitamin D3: Unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc. Natl. Acad. Sci. USA. 100 (25), 14754–14759.
Slominski A.T., Li W., Kim T.-K., Semak I., Wang J., Zjawiony J.K., Tuckey R.C. 2015. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 151, 25–37.
Sergeev G.V., Gilep A.A., Usanov S.A. 2014. The role of structural domains of cytochrome b5 in the interaction with cytochromes. Biokhimia (Rus.). 79 (5), 520–531.
Kaszuba M., McKnight D., Connah M.T., McNeil-Watson F.K., Nobbmann U. 2008. Measuring sub nanometre sizes using dynamic light scattering. J. Nanopart. Res. 10 (5), 823–829.
Kaluzhsky L.A., Ershov P.V., Kurpedinov K.S., Sonina D.S., Yablokov E.O., Shkel T.V., Gaiduke-vich I.V., Sergeev G.V., Usanov S A., Ivanov A.S. 2019. SPR analysis of protein-protein interactions involving cytochromes P450 and cytochrome b5 embedded in the bilayer lipid membrane. Biomed. Khimia (Rus.). 65 (5), 374–379.
Ershov P.V., Yablokov E.O., Florinskaya A.V., Mezentsev Y.V., Kaluzhskiy L.A., Tumilovich A.M., Gilep A.A., Usanov S.A., Ivanov A.S. 2019. SPR-based study of affinity of cytochrome P450s/redox partners interactions modulated by steroidal substrates. J. Steroid Biochem. Mol. Biol. 187, 124–129.
Šrejber M., Navrátilová V., Paloncýová M., Bazgier V., Berka K., Anzenbacher P., Otyepka M. 2018. Membrane-attached mammalian cytochromes P450: An overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J. Inorgan. Biochem. 183, 117–136.
Yamada A., Shimizu N., Hikima T., Takata M., Kobayashi T., Takahashi H. 2016. Effect of cholesterol on the Interaction of cytochrome P450 substrate drug chlorzoxazone with the phosphatidylcholine bilayer. Biochemistry. 55 (28), 3888–3898.
Neunzig J., Bernhardt R. 2014. Dehydroepiandrosterone sulfate (DHEAS) stimulates the first step in the biosynthesis of steroid hormones. PloS One. 9 (2), e89727.
Funding
The study of the CYP11A1–Adx interactions in the lipid bilayer membrane environment was performed with support of the Russian Foundation for Basic Research (project no. 19-04-00485). Optimization of SPR protocols for working with membrane proteins of P450-dependent systems was performed within the framework of the Program for Basic Research of State Academies of Sciences for 2013–2020. The work was performed using the equipment of the “Human Proteome” Core Facilities of the Institute of Biomedical Chemistry, supported by the Ministry of Education and Science (agreement no. 075-15-2019-1502 of September 5, 2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
The article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Dunina-Barkovskaya
Abbreviations: AdR, adrenodoxin reductase; Adx, adrenodoxin; BSA, bovine serum albumin; CPR, NADPH-dependent cytochrome-P450 oxidoreductase; CYB5B, mitochondrial cytochrome b5; CYP11A1, mitochondrial cytochrome P450 11A1 (P450scc); DLC, dynamic light scattering; DOPC, 1,2-dioleoyl-glycero-3-phosphatidylcholine; DOPG, 1, 2-dioleoyl-glycero-3-phosphoglycerol; EDC, 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimide hydrochloride; HBS, HEPES-buffered saline; NHS, N-hydroxy-succinimide; PPI, protein–protein interactions; RU, resonance units; SPR, surface plasmon resonance.
Rights and permissions
About this article
Cite this article
Ershov, P.V., Kaluzhskiy, L.A., Yablokov, E.O. et al. Application of the SPR Biosensor for the Analysis of Protein–Protein Interactions in Aqueous Environment and Bilayer Lipid Membrane As Exemplified by P450scc (CYP11A1). Biochem. Moscow Suppl. Ser. A 15, 89–96 (2021). https://doi.org/10.1134/S1990747821010049
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747821010049