Abstract—
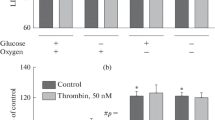
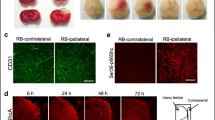
Thrombin is a multifunctional serine protease that attracts the attention of many researchers in particular in connection with a wide range of its effects in the nervous tissue. It is known that the main thrombin receptor is a protease-activated type 1 receptor, the functional activity of which is associated with adapter protein β-arrestin-2. Here we assessed potential involvement of β-arrestin-2 in the thrombin toxicity both in vitro and in vivo using gene knockout mice. It was found that thrombin induced dose-dependent cell death of cultured β-arrestin-2–/– astrocytes 48 h after the exposure. In contrast, thrombin did not exert any effects on the survival of astrocytes from wild-type animals. The in vivo study showed that β-arrestin-2 gene knockout did not alter the severity of the focal photoinduced cerebral ischemia aftereffects, which could involve additional cell types and molecular mechanisms in nervous tissue damage. Our findings demonstrate for the first time the necessity of β-arrestin-2 for the survival of mouse astrocytes under the toxic action of thrombin. At the CNS level, however, further studies are required to determine the key targets of this protease in each cell types of the nervous tissue and to clarify the role of β-arrestin signaling in neuroprotection.





Similar content being viewed by others
REFERENCES
Suo Z., Citron B.A., Festoff B.W. 2004. Thrombin: A potential proinflammatory mediator in neurotrauma and neurodegenerative disorders. Curr. Drug Targets – Inflamm. Allergy.3, 105–114.
Gingrich M.B., Traynelis S.F. 2000. Serine proteases and brain damage – is there a link? Trends Neurosci.23, 399–407.
Gorbacheva L.R., Kiseleva E.V., Savinkova I.G., Strukova S.M. 2017. New conception of the influence of hemostatic proteases on inflammation, neurotoxicity, and tissue regeneration. Biochem.82, 1018–1032.
Ramachandran R., Hollenberg M. 2008. Proteinases and signalling: Pathophysiological and therapeutic implications via PARs and more. Br. J. Pharmacol.153, 263–282.
Traynelis S.F., Trejo J. 2007. Protease-activated receptor signaling: New roles and regulatory mechanisms. Curr. Opin. Hematol.14, 230–235.
Ossovskaya V.S., Bunnett N.W. 2004. Protease-activated receptors: Contribution to physiology and disease. Physiol. Rev.84, 579–621.
Roy R.V., Ardeshirylajimi A., Dinarvand P., Yang L., Rezaie A.R. 2016. Occupancy of human EPCR by protein C induces β-arrestin-2 biased PAR1 signaling by both APC and thrombin. Blood.128, 1884–1893.
Griffin J.H., Zlokovic B. V, Mosnier L.O. 2015. Activated protein C: Biased for translation. Blood.125, 2898–2908.
Reiter E., Ahn S., Shukla A.K., Lefkowitz R.J. 2012. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol.52, 179–199.
Whalen E.J., Rajagopal S., Lefkowitz R.J. 2011. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol.17, 126–139.
Premont R.T., Gainetdinov R.R. 2007. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol.69, 511–534.
Violin J.D., Lefkowitz R.J. 2007. β-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci.28, 416–422.
Sofroniew M.V., Vinters H.V. 2010. Astrocytes: Biology and pathology. Acta Neuropathol.119, 7–35.
Bohn L.M., Lefkowitz R.J., Gainetdinov R.R., Peppel K., Caron M.G., Lin F.-T. 1999. Enhanced morphine analgesia in mice lacking β-arrestin-2. Science.286, 2495–2498.
Ivanova A.E., Gorbacheva L.R., Strukova S.M., Pinelis V.G., Reiser G. 2014. Activated protein C and thrombin participate in the regulation of astrocyte functions. Biochem.8, 50–59.
Lee J.-K., Park M.-S., Kim Y.-S., Moon K.-S., Joo S.-P., Kim T.-S., Kim J.-H., Kim S.-H. 2007. Photochemically induced cerebral ischemia in a mouse model. Surg. Neurol.67, 620–625.
Franklin K.B., Paxinos G. 2001. Mouse brain in stereotaxic coordinates. 2nd ed. London, Acad. Press.
Baskin Y.K., Dietrich W.D., Green E.J. 2003. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J. Neurosci. Methods.129, 87–93.
Benedek A., Móricz K., Jurányi Z., Gigler G., Lévay G., Hársing L., Mátyus P., Szénási G., Albert M. 2006. Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res.6, 159–164.
Pialat J.-B., Cho T.-H., Beuf O., Joye E., Moucharaffie S., Langlois J.-B., Nemoz C., Janier M., Berthezene Y., Nighoghossian N., Desvergne B., Wiart M. 2007. MRI monitoring of focal cerebral ischemia in peroxisome proliferator-activated receptor (PPAR)-deficient mice. NMR Biomed.6, 335–342.
Kang H.-M., Sohn I., Park C. 2015. Use of indocyanine green for optical analysis of cortical infarcts in photothrombotic ischemic brains. J. Neurosci. Methods.248, 46–50.
Kim G.W., Lewen A., Copin J.-C., Watson B.D., Chan P.H. 2001. The cytosolic antioxidant, copper/zinc superoxide dismutase, attenuates blood–brain barrier disruption and oxidative cellular injury after photothrombotic cortical ischemia in mice. Neuroscience. 105, 1007–1018.
Schroeter M., Jander S., Stoll G. 2002. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: Characterization of inflammatory responses. J. Neurosci. Methods.117, 43–49.
Cotrina M.L., Lou N., Tome-Garcia J., Goldman J., Nedergaard M. 2017. Direct comparison of microglial dynamics and inflammatory profile in photothrombotic and arterial occlusion evoked stroke. Neuroscience. 343, 483–494.
Wang H., Ubl J.J., Reiser G. 2002. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia.37, 53–63.
Watson B.D., Dietrich W.D., Busto R., Wachtel M.S., Ginsberg M.D. 1985. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol.17, 497–504.
Xi G., Reiser G., Keep R.F. 2003. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? J. Neurochem.84, 3–9.
ACKNOWLEDGMENTS
The authors thank D.A. Gorbachev (Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences) and V.S. Vyushkov (Moscow Lomonosov State University) for the help in mouse genotyping and interpretation of the obtained data. The work was supported by the Russian Foundation for Basic Research (project no. 18-34-00977).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
All procedures were performed in accordance with Directive 2010/63/EU of the European Parliament and the Council of the European Union. Experimental protocols were approved by the Bioethics Committee of Moscow Lomonosov State University (protocol no. 2018-10-25-93-0-3).
Additional information
Translated by M. Galkov
Abbreviations: APC, activated protein C; BBB, blood–brain barrier; GPCRs, G-protein-coupled receptors; GRKs, kinases of G-protein-coupled receptors; MRI, magnetic resonance imaging; PARs, protease-activated receptors; PC, protein C; PCR, polymerase chain reaction; TTC, 2,3,5-triphenyltetrazolium chloride.
Rights and permissions
About this article
Cite this article
Galkov, M.D., Ivanova, A.E., Gulyaev, M.V. et al. The Influence of β-Arrestin-2 Gene Knockout in Mice on Survival of Cultured Astrocytes Exposed to Thrombin and on the Cerebral Thrombosis Aftereffects In Vivo. Biochem. Moscow Suppl. Ser. A 14, 17–23 (2020). https://doi.org/10.1134/S1990747819060060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747819060060