Abstract
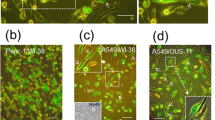
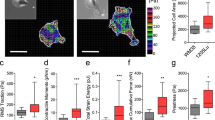
The abilities of tumor cells to invade and metastasize are frequent causes of death of cancer patients. Studying the mechanisms of cell motility alterations and acquisition of enhanced metastatic potential as the result of transformation is an important aspect in current cell biology. The initial and determinant step of cell motility is the formation of active cell edge with protrusions based on the Arp2/3-dependent actin polymerization. We used three different cell systems as examples of different models of tumor transformation to study the alteration and redistribution of protrusive activity caused by transformation in fibroblasts. We analyzed relationships between detected alterations and the acquisition of increased invasive potential by cells. Active edge of untransformed fibroblasts occupies about 50% of the cell perimeter and is concentrated at the cell front. There are well pronounced stable regions at the lateral cell edges. Tumor transformation causes redistribution of protrusive activity of fibroblasts irrespective of their origin and the nature of transforming agents. The length of active edges significantly increases, up to 92% of the total perimeter in fibrosarcoma cells of tumor origin. These cells have practically no stable edges. The intensity of protrusive activity of transformed cells is also increased. Single transformed cells show a decrease in the directionality and rate of migration on 2D substrate without special stimulation. Instead, they gain the capacity to migrate in 3D and to invade matrigel. These abilities increase in parallel with the intensification of edge activity. We showed that invasive abilities are not associated with the activation of matrix metalloproteinases in the studied cell systems. Our data demonstrate that the increase of length of active edge could be considered as an additional feature of cell transformation together with the reduction of stress fiber and focal adhesions and that the excessive protrusive activity results in the development of explorative migration of tumor cells.
Similar content being viewed by others
References
Hanahan D., Weinberg R.A. 2011. Hallmarks of cancer: The next generation. Cell. 144, 646–674.
Vasiliev J.M. 2004. Cytoskeletal mechanisms responsible for invasive migration of neoplastic cells. Int. J. Dev. Biol. 48, 425–439.
Friedl P., Wolf K. 2003. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer. 3, 362–374.
Vasiliev J.M., Gelfand I.M. 1981. Neoplastic and normal cells in culture. Cambridge: Cambridge University Press.
Kharitonova M.A., Kopnin P.B., Vasiliev J.M. 2007. Transformation by RAS oncogene decreases the width of substrate-spread fibroblasts but not their length. Cell. Biol. Int. 31, 220–223.
Minina S.A., Aleksandrova A.Y., Vasil’ev Y.M. 2003. Changes in the cell shape and actin cytoskeleton during the ras oncogene-induced transformation: A possible role of Rho kinase. Dokl. Akad. Nauk (Rus.). 388 (3), 413–415 [Translated version in: Dokl. Biol. Sci. 388, 83–85].
Lomakina M.E., Aleksandrova A.Yu. 2009. Analysis of changes induced by oncogene N-RAS expression in pattern and distribution of pseudopodial activity of fibroblasts. Ontogenez (Rus.). 40, 282–293.
Kopnin B.P. 2004. Fundamental properties of a neoplastic cell and basic mechanisms of their emergence. In: Kantserogenez (Carcinogenesis), D.G. Zaridze, Ed. Moscow: Meditsina, p. 86–102.
Huschtscha L.I., Holliday R. 1983. Limited and unlimited growth of SV40-transformed cells from human diploid MRC-5 fibroblasts. J. Cell. Sci. 63, 77–99.
Deryugina E.I. Bourdon M.A., Luo G.X., Reisfeld R.A., Strongin A. 1997. Matrix metalloproteinase-2 activation modulates glioma cell migration J. Cell. Sci. 110, 2473–2482.
Harvey D.M., Levine A.J. 1991. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 5, 2375–2385.
Jacobs J.P., Jones C.M., Baillie J.P. 1970. Characteristics of a human diploid cell designated MRC-5. Nature. 227, 168–170.
Rasheed S., Nelson-Rees W.A., Toth E.M., Arnstein P., Gardner M.B. 1974. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 33, 1027–1033.
Strongin A.Y., Marmer B.L., Grant G.A., Goldberg G.I. 1993. Plasma membrane-dependent activation of the 72-kDa type IVcollagenase is prevented by complex formation with TIMP. J. Biol. Chem. 268, 14033–14039.
Hinz B., Alt W., Johnen C., Herzog V., Kaiser H.W. 1999. Quantifying lamella dynamics of cultured cells by SACED, a new computer-assisted motion analysis. Exp. Cell Res. 251, 234–243.
Bear J.E., Svitkina T.M., Krause M., Schafer D.A., Loureiro J.J., Strasser G.A., Maly I.V., Chaga O.Y., Cooper J.A., Borisy G.G., Gertler F.B. 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 109, 509–521.
Young M.R., Liu S.W., Meisinger J. 2003. Protein phoshatase-2A restricts migration of Lewis lung carcinoma cells by modulating the phosphorylation of focal adhesion proteins. Int. J. Cancer. 103, 38–44.
Shutova M.S., Alexandrova A.Y., Vasiliev J.M. 2008. Regulation of polarity in cells devoid of actin bundle system after treatment with inhibitors of myosin II activity. Cell Motil. Cytoskeleton. 65, 734–746.
Svitkina T.M., Borisy G.G. 1999. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145, 1009–1035.
Pollard T.D. 2007. Regulation of actin filament assembly by Arp2/3 complex and formins. Biophys. Biomol. Struct. 36, 451–477.
Wolf K., Mazo I., Leung H., Engelke K., von Andrian U.H., Deryugina E.I., Strongin A.Y., Brö cker E.B., Friedl P. 2003. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 160, 267–277.
Sariahmetoglu M., Crawford B.D., Leon H., Sawicka J., Li L., Ballermann B.J., Holmes C., Berthiaume L.G., Holt A., Sawicki G., Schulz R. 2007. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 21, 2486–2495.
Yamazaki D., Kurisu K., Takenawa T. 2005. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 96, 379–386.
Rovensky, Y.A., Vasiliev, J.M. 2004. Morphogenetic reactions of cells and their perturbation during tumor transformation. In: Kantserogenez (Carcinogenesis), D.G. Zaridze, Ed. Moscow: Meditsina, pp. 376–414.
Dobrinskikh E.A. 2007. Study of roles of the microtubule systems and actin filaments in fibroblast motility. Extended Abstract of Cand. Sci. Dissertation. Moscow Lomonosov State University, Moscow, 2007, pp. 1–23.
Mercurio A.M., Rabinovitz I. 2001. Towards a mechanistic understanding of tumor invasion–leßsons from the a6ß4 integrin. Semin. Cancer Biol. 11, 129–141.
Ramos D.M., But M., Regezi J., Schmidt B.L., Atakilit A., Dang D., Ellis D., Jordan R., Li X. 2002. Expreßsion of integrin ß6 enchances invasive behavior in oral squamous cell carcinoma. Matrix Biol. 21, 297–307.
Maschler S., Wirl G., Spring H., Bredow D.V., Sordat I., Beug H., Reichmann E. 2005. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 17, 2032–2041.
Bar-Sagi D., Hall A. 2000. Ras and Rho GTPases: A family reunion. Cell. 103, 227–238.
Repasky G.A., Chenette E.J., Der C.J. 2004. Renewing the conspiracy theory debate: Does Raf function alone to mediate Ras oncogenesis? Trends Cell Biol. 14, 639–647.
Burridge K., Wennerberg K. 2004. Rho and Rac take center stage. Cell. 2, 167–179.
Ridley A.J. 2011. Life at the leading edge. Cell. 145, 1012–1022.
Pankov R., Endo Y., Even-Ram S., Araki M., Clark K., Cukierman E., Matsumoto K., Yamada K.M. 2005. A Rac switch regulates random versus directionally persistent cell migration. J. Cell Biol. 170, 793–802.
Chan C., Beltzner C.C., Pollard T.D. 2009. Cofilin dissociates Arp2/3-complex and branches from actin filaments. Curr. Biol. 19, 537–545.
DesMarais V., Ghosh M., Eddy R., Condeelis J. 2005. Cofilin takes the lead. J. Cell Sci. 118, 19–26.
Bershadsky A.D., Ballestrem C., Carramusa L., Zilberman Y., Gjlguin B., Khochbn S., Alexandrova A.Y., Verkhovsky A.B., Shemesh T., Kozlov M.M. 2006. Assembly and mechanosensory function of focal adhesions: Experiments and models. Eur. J. Cell Biol. 85, 165–173.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.E. Lomakina, M.S. Shutova, A.Y. Zhuravskaya, A.Y. Alexandrova, 2017, published in Biologicheskie Membrany, 2017, Vol. 34, No. 1, pp. 32–46.
Rights and permissions
About this article
Cite this article
Lomakina, M.E., Shutova, M.S., Zhuravskaya, A.Y. et al. Intensification and redistribution of protrusive activity is a feature of tumor transformation and is associated with an increase of the invasive potential of cells. Biochem. Moscow Suppl. Ser. A 11, 35–47 (2017). https://doi.org/10.1134/S1990747816040152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747816040152