Abstract
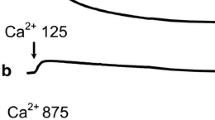
The kinetics of the processes accompanying the induction of Ca2+-dependent permeability (pore opening) of the inner membrane—swelling of organelles and Ca2+ release from the matrix—was studied in isolated liver mitochondria of mammals (mice, rats, and rabbits) and birds (pigeons and guinea fowls). It was found that the mitochondria of rats, pigeons, and guinea fowls of the gray-speckled population (GSP) are similar in terms of respiration and oxidative ATP synthesis, whereas mitochondria of rabbits exhibit a greater degree of coupling of respiration and ATP synthesis, and mitochondria of mice and Zagorskaya White breed (ZWB) guinea fowls, a lower degree of coupling. It was established that mammalian mitochondria energized by succinate oxidation and incubated with 1 mM of inorganic phosphate are able to swell upon the addition of 125 nmol of CaCl2 per 1 mg protein. Under these conditions, mitochondria of GSP and ZWB guinea fowls and pigeons are capable of swelling upon addition of at least 875, 875 and 1000 nmol of CaCl2 per 1 mg protein, respectively. Cyclosporin A (CsA, 1 μM) inhibits mitochondrial swelling. It was shown that mitochondria of mammalians and guinea fowls but not of pigeons are able to effectively absorb and retain Ca2+ in the matrix. Calcium retention capacity of mitochondria from rats, mice, rabbits, GSP, and ZWB guinea fowls were, respectively, 70, 57, 38, 844 and 793 nmol of CaCl2 per 1 mg of protein. In the presence of an oxidizing agent tert-butylhydroperoxide (TBH), the induction of the Ca2+-dependent pore in the mitochondria was observed upon addition of CaCl2 in substantially smaller quantities. TBH was most effective in the case of rabbit mitochondria and had the lowest efficiency in the case of guinea fowl and pigeon mitochondria.
Similar content being viewed by others
References
Saris N.E., Carafoli E. 2005. A historical review of cellular calcium handling, with emphasis on mitochondria. Biokhimia. (Rus.). 70, 231–239.
Lemasters J.J., Theruvath T.P., Zhong Z., Nieminen A.L. 2009. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 1787, 1395–1401.
Rasola A., and Bernardi P. 2011. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell. Calcium. 50, 222–233.
Zorov D.B., Juhaszova M., Sollott S.J. 2014. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950.
Gellerich F.N., Gizatullina Z., Gainutdinov T., Muth K., Seppet E., Orynbayeva Z., Vielhaber S. 2013. The control of brain mitochondrial energization by cytosolic calcium: The mitochondrial gas pedal. IUBMB Life. 65, 180–190.
Chalmers S., Nicholls D.G. 2003. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 278, 19062–19070.
Skulachev V.P., Bogachev A.V., Kasparinsky F.O. 2010. Membrannaja bioenergetika (Membrane bioenergetics). M.: Moscow University Press.
Siemen D., Ziemer M. 2013. What is the nature of the mitochondrial permeability transition pore and what is it not? IUBMB Life. 65, 255–262.
Malhi H., Guicciardi M.E., Gores G.J. 2010. Hepatocyte death: A clear and present danger. Physiol. Rev. 90, 1165–1194.
Zorov D.B., Plotnikov E.Y., Jankauskas S.S., Isaev N.K., Silachev D.N., Zorova L.D., Pevzner I.B., Pulkova N.V., Zorov S.D., Morosanova M.A. 2012. The phenoptosis problem: What is causing the death of an organism? Lessons from acute kidney injury. Biokhimia. (Rus.). 77, 893–906.
Skulachev V.P. 2012. What is “phenoptosis” and how to fight it? Biokhimia. (Rus.). 77, 827–846.
Leung A.W., Varanyuwatana P., Halestrap A.P. 2008. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 283, 26312–26323.
Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G.D., Petronilli V., Zoratti M., Szabó I., Lippe G., Bernardi P. 2013. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci USa. 110, 5887–5892.
Bonora M., Bononi A., De Marchi E., Giorgi C., Lebiedzinska M., Marchi S., Patergnani S., Rimessi A., Suski J.M., Wojtala A., Wieckowski M.R., Kroemer G., Galluzzi L., Pinton P. 2013. Role of the c subunit of the FOATP synthase in mitochondrial permeability transition. Cell Cycle. 12, 674–683.
Basso E., Petronilli V., Forte M.A., Bernardi P. 2008. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 283, 26307–26311.
Varanyuwatana P., Halestrap A.P. 2012. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion. 12, 120–125.
Kozhina O.V., Samartsev V.N. 2010. Uncoupling activity of fatty acids in liver mitochondria in the presence of substrates of ADP/ATPand aspartate/glutamate antiporters is increased under oxidative stress. Biol. Membrany (Rus.). 27, 184–188.
Ronchi J.A., Vercesi A.E., Castilho R.F. 2011. Reactive oxygen species and permeability transition pore in rat liver and kidney mitoplasts. J. Bioenerg. Biomembr. 43, 709–715.
Azzolin L., von Stockum S., Basso E., Petronilli V., Forte M.A., Bernardi P. 2010. The mitochondrial permeability transition from yeast to mammals. FEBS Lett. 584, 2504–2509.
Barja G. 2002. Rate of generation of oxidative stressrelated damage and animal longevity. Free Radic. Biol. Med. 33, 1167–1172.
Speakman J.R. 2005. Body size, energy metabolism and lifespan. J. Exp. Biol. 208, 1717–1730.
Hulbert A.J., Pamplona R., Buffenstein R., Buttemer W.A. 2007. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 87, 1175–1213.
Furness L.J., Speakman J.R. 2008. Energetics and longevity in birds. Age (Dordr). 30, 75–87.
Montgomery M.K., Hulbert A.J., Buttemer W.A. 2011. The long life of birds: The rat-pigeon comparison revisited. PLoS One. 6, e24138. doi: 10.1371/journalpone.0024138.
Vedernikov A.A., Dubinin M.V., Zabiakin V.A., Samartsev V.N. 2015. Ca2+-dependent nonspecific permeability of the inner membrane of liver mitochondria in the guinea fowl (Numida meleagris). J. Bioenerg. Biomembr. doi: 10.1007/s10863-015-9606-z.
Lukyanov A.S. 2008. Bioetika s osnovami bioprava: uchebnoe posobie (Bioethics with Grounds of Biolaws. A handbook). M.: Nauchnyi Mir.
Markova O.V., Bondarenko D.I., Samartsev V.N. 1999. The anion-carrier mediated uncoupling effect of dicarboxylic fatty acids in liver mitochondria depends on the position of the second carboxyl group. Biokhimia. (Rus.). 64, 679–685.
Hinkle P.C., Yu M.L. 1979. The phosphorus/oxygen ratio of mitochondrial oxidative phosphorilation. J. Biol. Chem. 254, 2450–2455.
Dubinin M.V., Adakeeva S.I., Samartsev V.N. 2013. Long-chain a,?-dioic acids as inducers of cyclosporin A-insensitive nonspecific permeability of the inner membrane of liver mitochondria loaded with calcium or strontium ions. Biokhimia. (Rus.). 78, 533–540.
Novgorodov S.A., Gudz T.I., Obeid L.M. 2008. Longchain ceramide is a potent inhibitor of the mitochondrial permeability transition pore. J. Biol. Chem. 283, 24707–24717.
Rolfe D.E. Brand M.D. 1997. The physiological significance of mitochondrial proton leak in animal cells and tissues. Biosci. Rep. 17, 9–16.
Brand M.D., Turner N., Ocloo A., Else P.L., Hulbert A.J. 2003. Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem. J. 376, 741–748.
Samartsev V.N., Polishchuk L.S., Paydyganov A.P., Zeldi I.P. 2004. Features of the uncoupling effect of fatty acids in liver mitochondria of mammals with different body weight. Biokhimia (Rus.). 69, 832–842.
Zabiyakin V.A. 2005. Polyvariability of pigmentation of fowl feathering. Ptitsevodstvo (Rus.). 10, 14–17.
Samartsev V.N., Vedernikov A.A., Dubinin M.V., Zabiyakin V.A. 2014. Comparative study of free oxidation in liver mitochondria of “wild”gray-speckled population and productive domestic breeds of guinea fowl (Numida meleagris). Zh. Evol. Biokhim. Fiziol. (Rus.). 50, 160–162.
Petronilli V., Cola C., Massari S., Colonna R., Bernardi P. 1993. Physiological effectors modify valtage sensing by the cyclosporine A-sensitive permeability transition pore of mitochondria. J. Biol. Chem. 268, 21939–21945.
Gostimskaya I.S., Grivennikova V.G., Zharova T.V., Bakeeva L.E., Vinogradov A.D. 2003. In situ assay of the intramitochondrial enzymes: use of alamethicin for permeabilization of mitochondria. Anal. Biochem. 313, 46–52.
Brustovetsky N., Brustovetsky T., Jemmerson R., Dubinsky J.M. 2002. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 80, 207–218.
Murphy A.N., Bredesen D.E., Gortopassi G., Wang E., Fiskum G. 1996. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc. Natl. Acad. Sci. USA. 93, 9893–9898.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.V. Dubinin, A.A. Vedernikov, E.I. Khoroshavina, S.I. Adakeeva, V.N. Samartsev, 2015, published in Biologicheskie Membrany, 2015, Vol. 32, No. 5–6, pp. 328–337.
Rights and permissions
About this article
Cite this article
Dubinin, M.V., Vedernikov, A.A., Khoroshavina, E.I. et al. Induction of calcium-dependent nonspecific permeability of the inner membrane in liver mitochondria of mammals and birds: A comparative study. Biochem. Moscow Suppl. Ser. A 10, 19–27 (2016). https://doi.org/10.1134/S1990747815050037
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747815050037