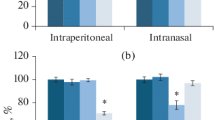
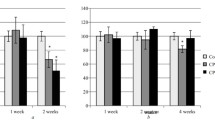
Abstract—The effects of noopept (1 mg/kg/day) and semax (0.6 mg/kg/day) on ex vivo [3H]-muscimol binding to GABAA-receptors in the prefrontal cortex of inbred BALB/c and C57BL/6 mice were examined after subchronic intraperitoneal (i.p.) and intranasal (i.n.) administration known to produce nootropic and anxiolytic activity in BALB/c but not in C57BL/6 mice. It was found that the initial specific binding (Bmax) and dissociation constants (Kd) for GABAA-receptors do not differ significantly between the two strains. However, in BALB/c mice i.p. noopept and semax administration increased GABAA-receptor density by 38 and 68% compared to the control group whereas after i.n. administration the increase was by 18 and 37%, respectively, which is in accord with the data on a more pronounced anxiolytic effect of i.p. peptide administration than i.n. administration. However, none of the drugs exerted a significant effect on the characteristics of GABAA-receptors in C57BL/6 mice, except for noopept, which decreased the Bmax value by 20% after i.p. administration. The similarity of noopept and semax effects on the GABAA-receptor density for both administration routes as well as the correlation of these changes with the intensity of anxiolytic effects depending on the administration route may indicate the involvement of GABAA-receptors in the mechanisms underlying the anxiolytic effects of these peptides.


Similar content being viewed by others
REFERENCES
Mironov, A.N., The Guidelines for Preclinical and Clinical Studies of Drugs. Part 1, Moscow: Grif i K, 2012.
Kovalev, G.I., Firstova, Yu.Yu., Salimov, R.M., and Kondrakhin, E.A., Psikhiatriya, 2010, vol. 45, no. 3, pp. 23–27.
Vasil’eva, E.V., Salimov, R.M., and Kovalev, G.I., Experimental and Clinical Pharmacology, 2012, vol. 75, no. 7, pp. 32–37.
Kovalev, G.I., Kondrakhin, E.A., Salimov, R.M., and Neznamov, G.G., Experimental and Clinical Pharmacology, 2013, vol. 76, no. 9, pp. 3–10.
Kovalev, G.I., Kondrakhin, E.A., Salimov, R.M., and Neznamov, G.G., Experimental and Clinical Pharmacology, 2014, vol. 77, no. 12, pp. 49–55.
Kovalev, G.I., Firstova, Yu.Yu., and Salimov, R.M., Experimental and Clinical Pharmacology, 2007, vol. 71, no. 12, pp. 12–17.
Kovalev, G.I. and Firstova, Yu.Yu., Klin. Farmakol. Ther., 2010, vol. 19, no. 6, pp. 72–73.
Kovalev, G.I., Vasil’eva, E.V., and Salimov, R.M., Zhurnal VND, 2019, vol. 69, no. 1, pp. 123–130.
Vasileva, E.V., Kondrakhin, E.A., Abdullina, A.A., Salimov, R.M., and Kovalev, G.I., Neurochem. J., 2020, vol. 14, no. 3, pp. 268–278.
Kondratenko, R.V., Derevyagin, V.I., and Skrebitsky, V.G., Neurisci. Lett., 2010, vol. 476, pp. 70–73.
Sharonova, I.N., Bukanova, Yu.V., Myasoedov, N.F., and Skrebitskii, V.G., Bull. Exp. Biol. Med., 2018, vol. 164, no. 5, pp. 612–616.
Haefely, W., Kulcsar, A., and Mohler, H., Psychopharmacol. Bull, 1975, vol. 11, pp. 58–59.
Smith, K.S., Engin, E., Meloni, E.G., and Rudolph, U., Neuropharmacology, 2012, vol. 63, pp. 250–258.
Glowinski, L.L. and Iversen, J., J. Neurochem., 1966, vol. 13, no. 8, pp. 655–669.
Vasil’eva, E.V., Kondrakhin, E.A., Salimov, R.M., and Kovalev, G.I., Experimental and Clinical Pharmacology, 2016, vol. 79, no. 9, pp. 3–11.
Vasil’eva, E.V., Salimov, R.M., and Kovalev, G.I., Farmakokinetika i Farmakodinamika, 2016, no. 2, pp. 31–36.
Ito, Y., Lim, D.K., Hayase, Y., Murakoshi, Y., and Ho, I.K., Neurochemical Research, vol. 17, no. 4, pp. 307–313.
Hawkinson, J., Acosta-Burruel, M., Kimbrough, C.L., Goodnough, D.B., and Wood, P.L., Eur. J. Pharmacol., 1996, vol. 304, nos. 1–3, pp. 141–146.
Firstova, Yu.Yu., Vasil’eva, E.V., and Kovalev, G.I., Experimental and Clinical Pharmacology, 2010, vol. 74, no. 1, pp. 6–10.
Seredenin, S.B., Voronina, T.A., Neznamov, G.G., Blednov, Yu.A., Badyshtov, B.A., Viglinskaya, I.V., Kozlovskaya, M.M., Kolotilinskaya, N.V., Yarkova, M.A., Savel’ev, V.L., Garibova, T.L., and Val’dman, E.A., Vestn. Ross. Akad. Med. Nauk, 1998, vol. 11, pp. 3–9.
Voronina, T.A. and Seredenin, S.B., Experimental and Clinical Pharmacology, 2002, vol. 65, no. 5, pp. 4–17.
Rudolph, U. and Mohler, H., ARPTDI, 2004, vol. 44, pp. 475–498.
Funding
The study was performed as part of State Assignment no. 0521-2019-0009 “Analyzing receptor mechanisms and searching for CNS protection drugs in cerebrovascular and cognitive disorders.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare no conflict of interests.
Ethical approval. The experiments were organized in compliance with ethical standards controlling experiments on animals (European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes: EST no. 123 of March 18, 1986, Strasbourg; Good Laboratory Practice of the Health Ministry of the RF no. 199n of April 1, 2016). The experiments were approved by the Bioethics commission of the Zakusov Institute of Pharmacology
Rights and permissions
About this article
Cite this article
Vasil’eva, E.V., Abdullina, A.A. & Kovalev, G.I. Subchronic Administration of Noopept and Semax Peptides Increases the Density of Cortical GABAA-Receptors in the Brain of BALB/c Mice. Neurochem. J. 15, 260–265 (2021). https://doi.org/10.1134/S1819712421030120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819712421030120