Abstract
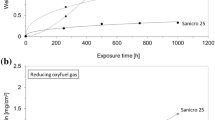
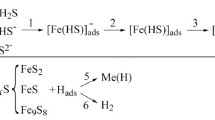
The composition of volatile and solid products of oxidation of hydrogen sulfide and stainless steel in gas mixtures containing H2S, O2, H2O, and CO2 has been determined using mass spectrometry, x-ray diffraction analysis, and scanning electron microscopy. It has been shown that holding an H2S–O2 mixture at 301 K results in prevailing formation of elemental sulfur and iron sulfides in the form of porous hygroscopic crust on the reactor wall surface. Formation of gas-phase sulfur causes self-acceleration of the oxidation of hydrogen sulfide; the resulting water triggers corrosion of the reactor wall. Heating of the resulting sulfur-sulfide crust in O2 medium is accompanied by formation of SO2 and heat release at T > 508 K. After heating of the H2S–CO2 mixture to 615 K, H2 and COS were found in the volatile reactants; no noticeable corrosion of the reactor wall has been detected. It has been established that addition of O2 to the H2S–CO2 mixture and its heating to 673 K leads to formation of ferrous sulfates. The mechanisms of the observed processes are discussed.
Similar content being viewed by others
References
Selim, H., Gupta, A.K., and Al Shoabi, A., Effect of Reaction Parameters on the Quality of Captured Sulfur in Claus Process, Appl. En., 2013, vol. 104, pp. 772–776.
Babich, I.V. and Moulijn, J.A., Science and Technology of Novel Process for Deep Desulfurization of Oil Refinery Streams: A Review, Fuel, 2003, vol. 82, pp. 607–631.
Corsi, R., Scaling and Corrosion in Geothermal Equipment: Problems and Preventive Measures, Geothermic, 1986, vol. 15, nos. 5/6, pp. 839–856.
Barbier, E., Geothermal Energy Technology and Current Status: An Overview, Ren. Sust. En. Rev., 2002, vol. 6, nos. 1/2, pp. 3–65.
Ouali, S., Chader, S., Belhamel, M., and Benziada, M., The Exploitation of Hydrogen Sulfide for Hydrogen Production in Geothermal Areas, Int. J. Hydr. En., 2011, vol. 36, pp. 4103–4109.
Reddy, E.L., Biju, V.M., and Subrahmanyam, C., Hydrogen Production from Hydrogen Sulfide in a Packed-Bed DBD Reactor, Int. J. Hydr. En., 2012, vol. 37, pp. 8217–8222.
Fedyaeva, O.N., Antipenko, V.R., Dubov, D.Yu., Kruglyakova, T.V., and Vostrikov, A.A., Non-Isothermal Conversion of the Kashpir Sulfur-Rich Oil Shale in a Supercritical Water Flow, J. Supercrit. Fluids, 2016, vol. 109, pp. 157–165.
Fedyaeva, O.N., Vostrikov, A.A., Shishkin, A.V., Sokol, M.Y., Fedorova, N.I., and Kashirtsev, V.A., Hydrothermolysis of Brown Coal in Cyclic Pressurization–Depressurization Mode, J. Supercrit. Fluids, 2012, vol. 62, pp. 155–164.
Vostrikov, A.A., Dubov, D.Yu., Sokol, M.Ya., Shishkin, A.V., and Fedyaeva, O.N., Brown Coal Gasification in Combustion in Supercritical Water, J. Eng. Therm., 2016, vol. 25, no. 1, pp. 55–66.
Vostrikov, A.A., Fedyaeva, O.N., and Kolobov, V.I., Conversion of Tar in Supercritical Water/Oxygen Fluid with Soot Suppression, J. Eng. Therm., 2017, vol. 26, no. 1, pp. 1–9.
Fedyaeva, O.N., Antipenko, V.R., and Vostrikov, A.A., Conversion of Sulfur-Rich Asphaltite in Supercritical Water and Effect ofMetal Additives, J. Supercrit. Fluids, 2014, vol. 88, pp. 105–116.
Fedyaeva, O.N. and Vostrikov, A.A., The Products of Heavy Sulfur-Rich Oil Conversion in a Counter Supercritical Water Flow and Their Desulfurization by ZnO Nanoparticles, J. Supercrit. Fluids, 2016, vol. 111, pp. 121–128.
Vostrikov, A.A., Fedyaeva, O.N., Shishkin, A.V., Dubov, D.Yu., and Sokol, M.Ya., Conversion of Municipal Sewage Sludge in Supercritical Water, Solid Fuel Chem., 2008, vol. 42, no. 6, pp. 384–393.
Fedyaeva, O.N., Vostrikov, A.A., Sokol, M.Ya., and Shatrova, A.V., Zinc Sulfidation by H2S and H2S/H2O Supercritical Fluids: Synthesis of Nanoparticles and Catalytic Effect of Water, J. Supercrit. Fluids, 2014, vol. 95, pp. 669–676.
Tsay, L.W., Lin, Y.J., and Chen, C., The Effect of Rolling Temperature and Sensitization Treatment on the Sulfide Stress Corrosion Cracking of 304L Stainless Steel, Corr. Sci., 2012, vol. 63, pp. 267–274.
Ning, J., Zheng, Y., Young, D., Brown, B., and Nesic, S., A Thermodynamic Study of Hydrogen Sulfide Corrosion of Mild Steel, Proc. NACE Int. Conf. on Corrosion, 2013, Paper no. 2462.
Bai, P., Zhao, H., Zheng, S., and Chen, C., Initiation and Development Stages of Steel Corrosion in Wet H2S Environments, Corr. Sci., 2015, vol. 93, pp. 109–119.
Xu, S., Huang, S., Guo, D., Zhao, Y., and Song, M., Failure Analysis of a Carbon Steel Pipeline to Wet Hydrogen Sulfide Environment, Eng. Failure An., 2017, vol. 71, pp. 1–10.
Bai, P., Zheng, S., Zhao, H., Ding, Y., Wu, J., and Chen, C., Investigations of theDiverseCorrosionProducts on Steel in a Hydrogen Sulfide Environment, Corr. Sci., 2014, vol. 87, pp. 397–406.
Zhao, W., Zou, Y., Matsuda, K., and Zou, Z., Corrosion Behavior of Reheated CGHAZof X80 Pipelines Steel in H2S-Containing Environments, Mat. Des., 2016, vol. 99, pp. 44–56.
Fatah, M.C., Ismail, M.C., Ari-Wahjoedi, B., and Kurnia, K.A., Effect of Sulfide Ion on the Corrosion Behavior of X52 Steel in a Carbon Dioxide Environment at Temperature 40 C, Mater. Chem. Phys., 2011, vol. 127, pp. 347–352.
Wang, P., Wang, J., Zheng, S., Qi, Y., Xiong, M., and Zheng, Y., Effect of H2S/CO2 Partial Pressure Ratio on the Tensile Properties of X80 Pipeline Steel, Int. J. Hydr. En., 2015, vol. 40, pp. 11925–11930.
Ming, Q., Jianfeng, L., Songying, C., and Yanpeng, Q., Experimental Study on Stress Corrosion Crack Propagation Rate of FV520B in Carbon Dioxide and Hydrogen Sulfide Solution, Res. Phys., 2016, vol. 6, pp. 365–372.
Sun, J., Sun C., Zhang, G., Li, X., Zhao, W., Jiang, T., Liu, H., Cheng, X., and Wang, Y., Effect of O2 and H2S Impurities on the Corrosion Behavior of X65 Steel inWater-Saturated Supercritical CO2 System, Corr. Sci., 2016, vol. 107, pp. 31–40.
Fedyaeva, O.N. and Vostrikov, A.A., Transformations of Pyrite and Pyrrhotite in Supercritical Water, Supercrit. Fluids: Theory Pract., 2016, vol. 11, no. 2, pp. 28–38.
Lidin, R.A., Andreeva, L.L., and Molochko, V.A., Konstanty neorganicheskikh veshchestv: spravochnik (Constants of Inorganic Substances: Handbook), Moscow: Drofa, 2006.
Vostrikov, A.A., Fedyaeva, O.N., Shishkin, A.V., Sokol, M.Ya., Kolobov, F.I., and Kolobov, V.I., Partial and CompleteMethane Oxidation in Supercritical Water, J. Eng. Therm., 2016, vol. 25, no. 4, pp. 474–484.
Lemmon, E.W., McLinden, M.O., and Freid, D.G., Thermophysical Properties of Fluid Systems. NIST Chemistry WebBook, NIST Standard Reference Database no. 69, Linstrom, P.J. and Mallard, W.G., Eds., Gaithersburg MD: National Institute of Standards and Technology, 2016, 20899; http://webbook.nist.gov/chemistry/fluid/.
Powder Diffraction File, PDF-4+, Rel. 2012, http://www.icdd.com/products/pdf14.htm.
Ampornrat, P. and Was, G.S., Oxidation of Ferric-Martensitic Alloys T91, HCM12A and HT-9 in Supercritical Water, J. Nucl. Mater., 2007, vol. 371, pp. 1–17.
U.S. Coast Guard, Chemical Hazard Response Information System (CHRIS)-Hazardous Chemical Data, Commandant Instruction 16465.12C. Washington, D.C.: U.S. Government Printing Office, 1999. http://www.uscg.mil/directives/cim/16000-16999/CIM_16465_12C.pdf.
Rhodes, C., Riddel, S.A., West, J., Williams, B.P., and Hutchings, G.J., The Low-Temperature Hydrolysis of Carbonyl Sulfide and Carbon Disulfide: A Review, Catal. Today, 2000, vol. 59, nos. 2/3, pp. 443–464.
NIST Mass Spectrometry Data Center, 2016, http://webbok.nist.gov/chemistry/form-ser.html.
Shaw, S.C., Groat, L.A., Jambor, J.L., Blowes, D.W., Hanton-Fong, C.J., and Stuparyk, R.A., Mineralogical Study of Base Metal Tailings with Various Sulfide Contents, Oxidized in Laboratory Columns and Field Lysimeters, Envir. Geol., 1998, vol. 33, nos. 2/3, pp. 209–217.
Steger, H.F., Oxidation of Sulfide Minerals: VII. Effect of Temperature and Relative Humidity on the Oxidation of Pyrrhotite, Chem. Geol., 1982, vol. 35, nos. 3/4, pp. 281–295.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vostrikov, A.A., Fedyaeva, O.N., Shishkin, A.V. et al. Oxidation of hydrogen sulfide and corrosion of stainless steel in gas mixtures containing H2S, O2, H2O, and CO2 . J. Engin. Thermophys. 26, 314–324 (2017). https://doi.org/10.1134/S181023281703002X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S181023281703002X