Abstract
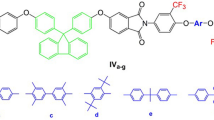
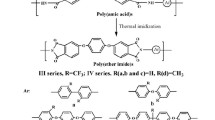
Balanced high transparency, thermal properties and organosolublility are urgent requirements for preparing polyimides. In this study, a series of fluorinated polyimides (Va-f) containing a multi-ether fluorinated diamine unit were designed. The corresponding PIs were synthesized through traditionally thermally polycondensation with bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]ether (II) and various commercial aromatic dinahydrides (IIIa-f). In particular, there are four rigid benzene rings and three flexible ether linkages in its fluorinated diamine structure (II), which is expected to achieve a balance in organic solubility and thermal properties. Va-f had inherent viscosity ranging from 0.88 to 1.24 dL/g. In addition, these fluorinated PIs exhibited high organo-solubility in common organic solvents and mechanical properties of tensile strengths of 92–116 MPa, tensile moduli of 2.0–42.3 GPa, elongations at break of 10–36%. Meanwhile, they also exhibited glass transition temperatures (Tgs) of 215–265°C, 10 wt % weight-loss temperatures of 525–597°C in N2. Among these PI films, Vf appeared excellent optical transparency, which displayed the cut-off wavelength at 361 nm with b* value of 8.7. For a comparative study, nonfluorinated analogous polyimides VIa-f were also synthesized. As a result, the fluorinated V series showed better organo-solubility, better optical property, lower dielectric constant and lower moisture absorption but without scarified thermal properties. Such attractive comprehensive properties make these fluorinated PIs as potential candidates for application in microelectronics industry.








Similar content being viewed by others
REFERENCES
X. Ren, Y. Zhang, Y. Liu, C. Yang, S. Dai, X. Wang, and J. Liu, Polymers 14, 3881 (2022).
S. Lee, Y. J. Cho, B. Han, J. Lee, S. Choi, T. Kang, H. Y. Chu, J. Kwag, S. C. Kim, and J. Jang, Adv. Eng. Mater. 24, 2100910 (2022).
H. Sugiyama, S. Sato, and K. Nagai, Polym. Adv. Technol. 33, 2113 (2022).
Y. Wang, Q. Chen, G. Zhang, Y. Wang, Z. Zhang, J. Fang, C. Zhao, and W. Li, Chem. Eng. J. 451, 138612 (2023).
S. Kim, Y. Lee, J. Park, Y. So, H. T. Jung, M. J. Ko, J. C. Won, S. Jeong, and Y. H. Kim, ACS Appl. Mater. Interfaces 15, 4408 (2023).
A. M. Orlova, A. Yu. Alentiev, T. I. Kolesnikov, A. Yu. Tsegelskaya, K. Z. Monakhova, S. V. Chirkov, R. Yu. Nikiforov, I. G. Abramov, and A. A. Kuznetsov, Polymer 256, 125258 (2022).
Y. Y. Liu, Y. K. Wang, and D. Y. Wu, J. Appl. Polym. Sci. 139, e52604 (2022).
P. K. Tapaswi and C. S. Ha, Macromol. Chem. Phys. 220, 1800313 (2019).
A. I. Wozniak, and A. S. Yegorov, V. S. Ivanov, S. M. Igumnov, and K. V. Tcarkova, J. Fluor. Chem. 180, 45 (2015).
X. J. Liu, M. S. Zheng, G. Chen, Z. M. Dang, and J. W. Zha, Energy Environ. Sci. 15, 56 (2022).
P. Ma, C. Dai, H. Wang, Z. Li, H. Liu, W. Li, and C. Yang, Compos. Commun. 16, 84 (2019).
Y. B. Zhuang, J. G. Seong, and Y. M. Lee, Prog. Polym. Sci. 92, 35 (2019).
C. Yi, W. Li, S. Shi, K. He, P. M, M. Chen, and C. Yang, Sol. Energy 195, 340 (2020).
I. Kaya and M. Kamaci, J. Appl. Polym. Sci. 135, 46573 (2018).
S. A. Tharakan and S. Muthusamy, RSC Adv. 11, 16645 (2021).
M. Zhong, X. Wu, C. Shu, Y. Wang, X. Huang, and W. Huang, React. Funct. Polym. 169, 105065 (2021).
I. A. Novakov, B. S. Orlinson, D. V. Zavyalov, S. V. Mednikov, E. N. Savelyev, E. A. Potaenkova, M. A. Nakhod, A. M. Pichugin, A. V. Kireeva, and M. N. Kovaleva, Russ. Chem. Bull. 70, 2457 (2021).
C. Wang, B. Yu, C. Jiang, X. Zhao, J. Li, and Q. Ren, Polym. Bull. 77, 6509 (2020).
X. Yan, F. Dai, Z. Ke, K. Yan, C. Chen, G. Qian, and H. Li, Eur. Polym. J. 164, 110975 (2022).
X. He, S. Zhang, Y. Zhou, F. Zheng, and Q. Lu, Polymer 254, 125073 (2022).
T. Zhang, Y. Pan, C. Song, B. Huang, and Z. Z. Huang, Polym. Bull. 77, 4077 (2020).
Y. Li, G. Sun, Y. Zhou, G. Liu, J. Wang, and S. Han, Prog. Polym. Sci. 172, 107103 (2022).
J. J. He, H. X. Yang, F. Zheng, and S. Y. Yang, Polymers 14, 649 (2022).
W. Q. Zhang, X. L. Wang, G. C. Liu, L. Chen, and Y. Z. Wang, RSC Adv. 6, 84284 (2016).
Y. Xu, M. Zhang, Y. Pang, T. Zheng, L. Zhang, Z. Wang, and J. Yan, Eur. Polym. J. 179, 111528 (2022).
K. Xie, S. Y. Zhang, J. G. Liu, M. H. He, and S. Y. Yang, J. Polym. Sci., Part A: Polym. Chem. 39, 2581 (2001).
W. Jang, D, Shin, S. Choi, S. Park, and H. Han, Polymer 48, 2130 (2007).
M. J. Hu, H. Q. Chen, M. X. Wang, G. Liu, C. H. Chen, G. T. Qian, and Y. H. Yu, J. Polym. Sci. 59, 329 (2021).
H. Zheng, C. Y. Wang, Y. Ma, W. Tao, X. Y. Zhao, J. Li, and Q. Ren, J. Macromol. Sci. Pure. Appl. Chem. 58, 880 (2021).
C. P. Yang and F. Z. Hsiao, J. Polym. Res. 10, 181 (2003).
Q. Wu, X. Ma, F. Zheng, X. Lu, and Q. Lu, Polym. Int. 68, 1186 (2019).
K. M. Jeong, P. K. Tapaswi, T. Kambara, and R. Ishige, High Perform. Polym. 32, 620 (2020).
C. P. Yang and Y. C. Chen, J. Appl. Polym. Sci. 96, 2399 (2005).
A. I. Barzic, C. Hulubei, M. Asandulesa, G. Lisa, D. Popovici, I. Stoica, A. Nicolescu, and R. M. Albu, Polym. Test. 90, 106704 (2020).
X. Wu, J. Cai, and Y. Cheng, J. Appl. Polym. Sci. 139, 51972 (2022).
Funding
Financial support from the National Science and Technology Council, Taiwan is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yung-Chung Chen, Su, YY. & Chiang, HC. Organosoluble and Light-Colored Fluorinated Polyimides Prepared from Bis[4-(4-amino-2-trifluoronethylphenoxy)phenyl]ether and Aromatic Dianhydrides. Polym. Sci. Ser. B 65, 514–527 (2023). https://doi.org/10.1134/S1560090423701129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090423701129