Abstract
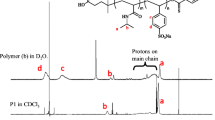
The diblock and triblock copolymers were synthesized by chain extending on poly(N-isopropyl-acrylamide) (H-PNIPA) with HO–CH2CH2– and α-Cl end group obtained from atom transfer radical polymerizaiton technique by aid of the same technique, using ethylmethacrylate (EMA), 2-hydroxypropyl methacrylate (HPMA), N,N-dimethylacrylamide (DMA) and benzylmethacrylate (BMA) as comonomers. Number average molecular weight of H-PNIPA homopolymer and the diblock copolymers, H-PNIPA-b-PEMA, H-PNIPA-b-PHPMA, H-PNIPA-b-PDMA were estimated as 1960, 3990, 3110 and 4040 g/mol, from 1H NMR spectra. Structure of the copolymers were characterized by FTIR and 1H NMR techniques. TGA curves showed that the thermal stability of the diblock and triblock copolymers decrease comparing to H-PNIPA. The Tg temperatures of the polymers, H-PNIPA, the diblock copolymers, and triblock copolymers, H-PNIPA-b-PDMA-b-PEMA and H-PNIPA-b-PDMA-b-PBMA were predicted from the DSC curves, as 130°C, in range of 105–115, and 90 and 100°C, respectively. The dielectric constant decreased slightly in the frequency range between 0.1 and 2.0 kHz at room temperature. Cloud point temperature (CPT) values of H-PNIPA homopolymer and the block copolymers were determined using UV–Vis, turbidimetry and viscometer techniques in aqueous solution. The CPTs found by three different techniques for each polymer were close to each other. In the diblock and triblock copolymers, some differences in CPTs were observed according to comonomer and these were discussed.






Similar content being viewed by others
REFERENCES
B. Jeong and A. Gutowska, Trends Biotechnol. 20, 305 (2002).
A. Kikuchi and T. Okano, Prog. Polym. Sci. 27, 1165 (2002).
N. A. Pattanashetti, G. B. Heggannavar, and M. Y. Kariduraganavar, Procedia Manuf. 12, 263 (2017)
Y. Qiu and K. Park, Adv. Drug Delivery Rev. 53, 321 (2001).
A. Hatefi and B. Amsden, J. Controlled Release 80, 9 (2002).
A. Klkuchi and T. Okano, Adv. Drug Delivery Rev. 54, 53 (2002).
R. A. Gemeinhart, J. Chen, H. Park, and K. Park, J. Biomater. Sci., Polym. Ed. 11, 1371 (2000).
M. Torres-Lugo, M. Garcie, R. Record, and N. A. Peppas, Biotechnol. Prog. 18, 612 (2002).
Y. Qiu and K. Park, Adv. Drug Deliverey Rev. 53, 321 (2001).
L. Y. Galaev and B. Mattiasson, Trends Biotechnol. 17, 335 (2000).
J. Kobayashi, A. Kikuchi, K. Sakai, and T. Okano, J. Chromatogr. A 958, 109 (2002).
S. Anastase-Ravion, Z. Ding, A. Pelle, A. S. Hoffman, and D. Letourneur, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 761, 247 (2001).
V. T. Pinkrah, M. J. Snowden, J. C. Mitchell, J. Seidel, B. Z. Chowdhry, and G. R. Fern, Langmuir 19, 585 (2003).
S. G. Eun and M. H. Samuel, Prog. Polym. Sci. 29, 1173 (2004).
L. Mario, B. Cyrille, P. Carmen, C. Teresa, W. Michael, T. Lei, and P. D. Thomas, J. Polym. Sci., Part A: Polym. Chem. 48, 2783(2010).
G. Arijit, P. Abhijit, O. S. Suma, and K. S. Kalyan, Asian J. Pharm. Sci. 10, 99 (2015).
D. C. Coughlan and O. I. Corrigan, J. Pharm. Sci. 97, 318 (2008).
D. C. Coughlan and O. I. Corrigan, Int. J. Pharm. 313, 163 (2006).
Y. K. Jhon, R. R. Bhat, C. Jeong, J. R. Orlando, S. Igal Szleifer, and G. Jan, Macromol. Rapid Commun. 27, 697 (2006).
N. Cheng, W. G. Liu, Z. Q. Cao, W. H. Ji, D. C. Liang, G. Guo, and J. Y. Zhang, Biomaterials, 27, 4984 (2006).
Y. M. Zhou, A. Ishikawa, R. Okahashi, K. Uchida, Y. Nemoto, M. Nakayama, and Y. Nakayama, J. Controlled Release 123, 239 (2007).
M. R. Aguilar, C. Elvira, A. Gallardo, B. Vázquez, and J. S. Román, “Smart Polymers and Their Applications as Biomaterials,” in Topics in Tissue Engineering, Vol. 3, Ed. by N. Ashammakhi, R. Reis, and E. Chiellini (Oulu Univ., Oulu, 2007), Chap. 6.
R. Ghizal, G. R. Fatima, and S. Srivastava, Int. J. Eng. Technol. Manage. Appl. Sci. 2, 104 (2014).
M. Heskins and J. E. Guillet, J. Macromol. Sci., Part A: Pure Appl. Chem. 2, 1441 (1968).
N. S. Reddy and K. S. V. K. Rao, Indian J. Adv. Chem. Sci. 4, 214 (2016).
A. Kumar, M. Kamihira, I. Y. Galaev, B. Mattiasson, and S. Iijima, Biotechnol. Bioeng. 75, 570 (2001).
N. A. Plate, L. I. Lebedeva, and L. I. Valuev, Polym. J. 31, 21 (1999).
J. C. Leroux, E. Roux, D. Garrec, K. Hong, and D. C. Drummond, J. Controlled Release 84, 72 (2001).
S. lboğa and M. Coşkun, Polym. Sci., Ser. B 56, 848 (2014).
Q. Duan, A. Narumi, Y. Miura, X. Shen, S. I. Sato, T. Satoh, and T. Kakuchi, Polym. J. 38, 306 (2006).
S. Furyk, Y. Zhang, D. Ortiz-Acosta, P. S. Cremer, and D. E. Bergbreiter, J. Polym. Sci., Part A: Polym. Chem. 44, 1492 (2006).
B. Ray, Y. Isobe, K. Matsumoto, S. Habaue, Y. Okamoto, M. Kamigaito, and M. Sawamoto, Macromolecules 37, 1702 (2004).
S. Furyk, Y. Zhang, D. Ortiz-Acosta, P. S. Cremer, and D. E. Bergbreiter, J. Polym. Sci., Polym. Chem. 44, 1492 (2006).
H. Feil, Y. H. Bae, J. Feijen and S. W. Kim, Macromolecules 26, 2496 (1993).
E. Hasan, M. Zhang, A. H. E. Müller and Ch. B. Tsvetanov, J. Macromol. Sci., Part A: Pure Appl. Chem. 41, 467 (2004).
P. Chen, J. Chen and Y. Cao, J. Macromol. Sci., Part A: Pure Appl. Chem. 50, 478 (2013).
G. Fan, J. Guo, M. Dong, and Y. Feng, J. Macromol. Sci., Part A: Pure Appl. Chem. 51, 881 (2014).
Spectrometric Identification of Organic Compounds, Ed. by R. M. Silverstein, F. X. Webster, and D. C. Kiemle, 7th ed. (J. Wiley and Sons, Inc., New York, 2005).
L. Feng, J. Hu, Z. Liu, F. Zhao, and G. Liu, Polymer 48, 3616 (2007).
J. Sheng, H. Chen, B. Li, and L. Chang, Appl. Phys. A: Mater. Sci. Process. 110, 511 (2013).
M. A. Ward and T. K. Georgiou, Polymers 3, 1215 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Serzen İlboğa, Pekdemir, E. & Coşkun, M. Cloud Point Temperature, Thermal and Dielectrical Behaviors of Thermosensitive Block Copolymers Based N-Isopropylacrylamide. Polym. Sci. Ser. B 61, 32–41 (2019). https://doi.org/10.1134/S1560090419010068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090419010068