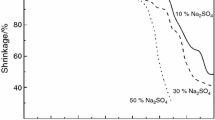
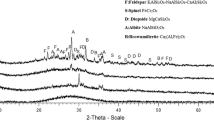
Abstract
X-ray diffraction and electron microscopy were used to examine phase composition and the distribution of elements in glass materials which simulated the vitrified high-level wastes. The variations in the compositions of forming glass materials due to the high content of iron in the waste (as well as the low contents of nickel and chrome) from sodium-alumina-phosphate to sodium-iron-phosphate caused variations in phase compositions and distributions of elements between the phases. Sodium-alumina-phosphate glass characterized by an increased tendency for crystallization which declined on the substitution of about half of all Al2O3 for Fe2O3 and NiO, and increased again with a subsequent increase in the content of oxides of transition elements. Chrome oxide(III) served as a crystallization catalyst. Substitution of 6 wt % P2O5 for B2O3 increased somewhat the crystallization durability of the glass containing a high amount of Al2O3, but produced no effect in the case of glass with a high content of iron and nickel oxides. The dependence of the hydrolytic durability of glass materials on composition was complicated, but, in general, the durability declined on transition from alumina-phosphate to iron-phosphate glass.
Similar content being viewed by others
References
Glagolenko, Yu.V., Drozhko, E.G., and Rovnyi, S.I., The main directions of the solution of environmental problems associated with the current and past activities of the Federal State Unitary Enterprise “Production Association Mayak,” Vopr. Radiats. Bezop., 2006, no. 1, pp. 23–34.
Stefanovsky, S.V., Yudintsev, S.V., Giere, R., and Lumpkin, G.R., Nuclear waste forms, energy, waste and the environment: A geological perspective, Geol. Soc. Spec. Publ., 2004, vol. 236, pp. 37–63.
Stefanovsky, S.V., Nikonov, B.S., and Marra, J.C., Characterization of the glass-ceramic material prepared upon vitrification of an iron-containing surrogate of high-level wastes in a cold crucible, Glass Phys. Chem., 2007, vol. 33, no. 6, pp. 576–586.
Stefanovsky, S.V., Nikonov, B.S., and Marra, J.C., Characterization of glassy materials for immobilization of radioactive waste with a high-iron oxide content, Glass Phys. Chem., 2008, vol. 34, no. 3, pp. 292–299.
Sales, B.C., Abraham, M.M., Bates, J.B., and Boatner, L.A., Structural properties of lead–iron phosphate glasses, J. Non-Cryst. Solids, 1985, vol. 71, pp. 103–112.
Sales, B.C. and Boatner, L.A., Physical and chemical characteristics of lead iron phosphate nuclear waste glass, J. Non-Cryst. Solids, 1986, vol. 79, pp. 83–116.
Day, D.E., Wu, Z., Ray, C.S., and Hrma, P., Chemically durable iron phosphate glass wasteforms, J. NonCryst. Solids, 1998, vol. 241, pp. 1–12.
Marasinghe, G.K., Karabulut, M., Ray, C.S., Day, D.E., Shuh, D.K., Allen, P.G., Saboungi, M.L., Grimsditch, M., and Haeffner, D., Properties and structure of vitrified iron phosphate nuclear wasteforms, J. Non-Cryst. Solids, 2000, vols. 263–264, pp. 146–154.
Kim, C.W., Ray, C.S., Zhu, D., Day, D.E., Gombert, D., Aloy, A.S., Moguš-Milankovi, A., and Karabulut, M., Chemically durable iron phosphate glasses for vitrifying sodium bearing waste (SBW) using conventional and cold crucible induction melting (CCIM) techniques, J. Nucl. Mater., 2003, vol. 322, pp. 152–164.
Kim, C.-W. and Day, D.E., Immobilization of Hanford LAW in iron phosphate glass, J. Non-Cryst. Solids, 2003, vol. 331, pp. 20–31.
GOST R (State Standard) 52126-2003: Radioactive Wastes: Determination of the Chemical Resistance of Solidified Highly Active Wastes Using the Method of Long-Term Leaching, 2004.
De la Rochère, M., Kahn, A., D’Yvoire, F., and Bretey, E., Crystal structure and cation transport properties of the ortho-diphosphates Na7(MP2O7)4PO4 (M = Al, Cr, Fe), Mater. Res. Bull., 1985, vol. 20, pp. 27–34.
Stefanovsky, S.V., Sorokaletova, A.N., and Nikonov, B.S., Phase composition and elemental partitioning in glass-ceramics containing high-Na/Al highlevel waste, J. Nucl. Mater., 2012, vol. 424, nos. 1–3, pp. 75–81.
Medvedev, G.M., Remizov, M.B., and Dubkov, S.A., Investigation of the properties of phosphate and boron phosphate glasses, Vopr. Radiats. Bezop., 2004, no. 2, pp. 15–23.
Medvedev, G.M., Remizov, M.B., Gilev, A.G., Dubkov, S.A., Korchenkin, K.K., and Mashkin, A.N., Investigation of the process of melting of boron phosphate glasses on an experimental electric furnace of direct electrical heating, Vopr. Radiats. Bezop., 2004, no. 4, pp. 3–10.
Oziraner, S.N., Minaev, A.A., Kuznetsov, D.G., and Prokhorova, N.P., Comparison of some properties of phosphate and silicate glasses intended for the vitrification of aluminum-containing radioactive wastes: Investigations in the field of detoxification of liquid, solid, and gaseous radioactive wastes and deactivation of contaminated surfaces, in Materialy IV nauchnotekhnicheskoi konferentsii SEV, Moscow, 1976 (Proceedings of the Fourth Scientific and Technical Conference of the Council for Mutual Economic Assistance, Moscow, Soviet Union, December 22–23, 1976), Moscow: Atomizdat, 1978, pp. 94–102.
Brezhneva, N.E., Minaev, A.A., and Oziraner, S.N., Vitrification of high sodiumaluminum wastes: composition ranges and properties, in Scientific Basis for Nuclear Waste Management, McCarthy, G.J., Ed., New York: Plenum, 1979, vol. 1, pp. 43–50.
Fosfatnye stekla s radioaktivnymi otkhodami (Phosphate Glasses with Radioactive Waste), Vashman, A.A. and Polyakov, A.S., Eds., Moscow: TsNIIAtominform, 1997.
Koval’skii, A.M., Martynov, K.V., Budantseva, N.A., Kotel’nikov, A.R., and Tananaev, I.G., Transformation of the structure of aluminophosphate glasses under the influence of temperature, in XI Molodezhnaya nauchnaya konferentsiya, IKhS im. I.V. Grebenshchikova RAN, Sankt-Peterburg, 2010 (Proceedings of the Eleventh Youth Scientific Conference, Grebenshchikov Institute of Silicate Chemistry of the Russian Academy of Sciences, St. Petersburg, Russia, December 9–10, 2010), St. Petersburg, 2010, pp. 79–81.
Martynov, K.V., Budantseva, N.A., Tananaev, I.G., Koval’skii, A.M., and Kotel’nikov, A.R., Experimental investigation of the influence of the modifier components on the devitrification of Na2O–Al2O3–P2O5 glasses at elevated temperatures, Vestn. Otd. Nauk Zemle Russ. Akad. Nauk, 2011, vol. 3, no. NZ6072. doi 10.2205/2011NZ000202
Berul’, S.I. and Voskresenskaya, N.K., Interaction of sodium metaphosphate and aluminum oxide, Zh. Neorg. Khim., 1968, vol. 13, no. 2, pp. 422–427.
Ul’yantsev, V.M. and Zholobova, L.S., Structure of glasses in the Na2O–Al2O3–P2O5 system, Izv. Akad. Nauk SSSR, Neorg. Mater., 1977, vol. 13, no. 8, pp. 1527–1528.
Hooper, A., McGeehin, P., Harrison, K.T., and Tofield, B.C., Ionic conductivity of pure and doped Na3PO4, J. Solid State Chem., 1978, vol. 24, pp. 265–275.
Saidi, M., Coffy, G., and Sibieude, F., Les systemes binaires AlPO4–M3PO4 (M = Li, Na, K), J. Therm. Anal., 1995, vol. 44, pp. 15–23.
Dollase, W.A., Merwin, L.H., and Sebald, A., Structure of Na3–3xAlxPO4, J. Solid State Chem., 1989, vol. 83, pp. 140–149.
Gusarov, V.V., Mikirticheva, G.A., Shitova, V.I., Grabovenko, L.Yu., and Kuchaeva, S.K., Phase relationships in the NaPO3–Al2O3 glass-forming system, Glass Phys. Chem., 2002, vol. 28, no. 5, pp. 309–316.
Pintard-Scrépel, M., D’Ivoire, F., and Rémy, F., Polymorphisme et conduction ionique du phosphate Na3Fe2(PO4)3, C. R. C. R. Acad. Sc. Paris Acad. Sci., Ser. C, 1978, vol. 286, pp. 381–383.
D’Yvoire, F. and Pintard-Scrépel, M., Hydroxyhydrogénosels: Le phosphate et l’arséniate non-stoechiométriques de formule idéale Na3Al(OH)(HXO4)(XO4) (X = P ou As), J. Solid State Chem., 1981, vol. 37, pp. 103–111.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.V. Stefanovsky, M.B. Remizov, E.A. Belanova, P.V. Kozlov, R.A. Makarovsky, O.I. Stefanovskaya, B.S. Nikonov, 2015, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Stefanovskya, S.V., Remizov, M.B., Belanova, E.A. et al. Phase composition, structure, and hydrolytic durability of phosphate glass materials for immobilizing liquid highly level waste rich in-iron-group elements. Glass Phys Chem 41, 489–499 (2015). https://doi.org/10.1134/S1087659615050193
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659615050193