Abstract
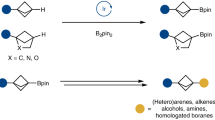
Cycloboration of methylidenecycloalkanes with PhBCl2 in the presence of Cp2TiCl2 as catalyst was performed for the first time to afford previously unknown spiro-boracarbocycles in 70–80% yields. The structure and properties of the obtained spiro-boriranes were studied by 11B, 1H, and 13C NMR and DOSY experiments. 1-Phenyl-substituted boraspiranes were found to be stable in solution at room temperature for 24 h.












Similar content being viewed by others
REFERENCES
Khusainova, L.I., Khafizova, L.O., Tyumkina, T.V., Ryazanov, K.S., and Dzhemilev, U.M., J. Organomet. Chem., 2017, vol. 832, p. 12. https://doi.org/10.1016/j.jorganchem.2017.01.009
Khusainova, L.I., Khafizova, L.O., Tyumkina, T.V., and Dzhemilev, U.M., Russ. J. Org. Chem., 2015, vol. 51, p. 1517. https://doi.org/10.1134/S1070428015110019
Khusainova, L.I., Khafizova, L.O., Tyumkina, T.V., and Dzhemilev, U.M., Russ. J. Gen. Chem., 2016, vol. 86, p. 1038. https://doi.org/10.1134/S1070363216060335
Khusainova, L.I., Khafizova, L.O., Tyumkina, T.V., Ryazanov, K.S., Popodko, N.R., and Dzhemilev, U.M., J. Organomet. Chem., 2018, vol. 873, p. 73. https://doi.org/10.1016/j.jorganchem.2018.08.005
Dzhemilev, U.M., Khusainova, L.I., Ryazanov, K.S., and Khafizova, L.O., Russ. Chem. Bull., Int. Ed., 2021, vol. 70, p. 1851. https://doi.org/10.1007/s11172-021-3292-2
Rao, Y.-L., Amarne, H., Zhao, S.-B., McCormick, T.M., Martić, S., Sun, Y., Wang, R.-Y., and Wang, S., J. Am. Chem. Soc., 2008, vol. 130, p. 12898. https://doi.org/10.1021/ja8052046
Baik, C., Hudson, Z.M., Amarne, H., and Wang, S., J. Am. Chem. Soc., 2009, vol. 131, p. 14549. https://doi.org/10.1021/ja906430s
Rao, Y.-L., Amarne, H., and Wang, S., Coord. Chem. Rev., 2012, vol. 256, p. 759. https://doi.org/10.1016/j.ccr.2011.11.009
Mellerup, S.K. and Wang, S., Sci. China Mater., 2018, vol. 61, p. 1249. https://doi.org/10.1007/s40843-018-9306-8
McFadden, T.R., Fang, Ch., Geib, S.J., Merling, E., Liu, P., and Curran, D.P., J. Am. Chem. Soc., 2017, vol. 139, p. 1726. https://doi.org/10.1021/jacs.6b09873
Dai, W., McFadden, T.R., Curran, D.P., Früchtl, H.A., and Walton, J.C., J. Am. Chem. Soc., 2018, vol. 140, p. 15868. https://doi.org/10.1021/jacs.8b09288
Bissinger, P., Braunschweig, H., Kraft, K., and Kupfer, T., Angew. Chem., Int. Ed., 2011, vol. 50, p. 4704. https://doi.org/10.1002/anie.201007543
Braunschweig, H., Claes, C., Damme, A., Deißenberger, A., Dewhurst, R.D., Hörl, C., and Kramer, T., Chem. Commun., 2015, vol. 51, p. 1627. https://doi.org/10.1039/c4cc09036e
Wehrmann, R., Klusik, H., and Berndt, A., Angew. Chem., Int. Ed., 1984, vol. 23, p. 369. https://doi.org/10.1002/anie.198403691
Klusik, H. and Berndt, A., Angew. Chem., Int. Ed., 1983, vol. 22, p. 877. https://doi.org/10.1002/anie.198308771
Pues, C., Baum, G., Massa, W., and Berndt, A., Z. Naturforsch., Teil B, 1988, vol. 43, p. 275. https://doi.org/10.1515/znb-1988-0307
Glaser, B., Mayer, E.P., Nöth, H., Rattay, W., and Wietelmann, U., Z. Naturforsch., Teil B, 1988, vol. 43, p. 449. https://doi.org/10.1515/znb-1988-0411
Balzereit, C., Kybart, C., Winkler, H.-J., Massa, W., and Berndt, A., Angew. Chem., Int. Ed., 1994, vol. 33, p. 1487. https://doi.org/10.1002/anie.199414871
Mayer, P. and Noth, H., Chem. Ber., 1993, vol. 126, p. 1551. https://doi.org/10.1002/cber.19931260708
Wrackmeyer, B., Annu. Rep. NMR Spectrosc., 1988, vol. 20, p. 61. https://doi.org/10.1016/s0066-4103(08)60170-2
Brown, H.C. and Zaidlewicz, M., J. Am. Chem. Soc., 1976, vol. 98, p. 4917. https://doi.org/10.1021/ja00432a037
Klebe, J.F., Finkbeiner, H., and White, D.M., J. Am. Chem. Soc., 1966, vol. 88, p. 3390. https://doi.org/10.1021/ja00966a038
Wilkey, J.D. and Schuster, G.B., J. Am. Chem. Soc., 1991, vol. 113, p. 2149. https://doi.org/10.1021/ja00006a037
Midland, M.M. and Brown, H.C., J. Am. Chem. Soc., 1973, vol. 95, p. 4069. https://doi.org/10.1021/ja00793a052
Sobota, P., Pluzinski, T., Jezowska-Trzebiatowska, B., and Rummel, S., J. Organomet. Chem., 1980, vol. 185, p. 69. https://doi.org/10.1016/s0022-328x(00)94401-2
Eisch, J.J., Boleslawski, M.P., and Tamao, K., J. Org. Chem., 1989, vol. 54, p. 1627. https://doi.org/10.1021/jo00268a025
Tomboulian, P., Amick, D., Beare, S., Dumke, K., Hart, D., Hites, R., Metzger, A., and Nowak, R., J. Org. Chem., 1973, vol. 38, p. 322. https://doi.org/10.1021/jo00942a026
Fitjer, L. and Quabeck, U., Synth. Commun., 1985, vol. 15, p. 855. https://doi.org/10.1080/00397918508063883
Wittig, G. and Schoellkopf, U., Org. Synth., 1960, vol. 40, p. 66. https://doi.org/10.15227/orgsyn.040.0066
Barluenga, J., Fernández-Simón, J.L., Concellón, J.M., and Yus, M., J. Chem. Soc., Perkin Trans. 1, 1988, p. 3339. https://doi.org/10.1039/p19880003339
Lebel, H., Davi, M., Díez-González, S., and Nolan, S.P., J. Org. Chem., 2007, vol. 72, p. 144. https://doi.org/10.1021/jo061781a
Masuda, Y., Ikeshita, D., and Murakami, M., Helv. Chim. Acta, 2021, vol. 104, article ID e2000228. https://doi.org/10.1002/hlca.202000228
Kobayashi, S., Kawamoto, T., Uehara, S., Fukuyama, T., and Ryu, I., Org. Lett., 2010, vol. 12, p. 1548. https://doi.org/10.1021/ol1002847
ACKNOWLEDGMENTS
Spectral studies were performed at the Agidel regional joint center (Ufa Federal Research Center, Russian Academy of Sciences).
Funding
This study was financially supported in the framework of state assignment no. FMRS-2022-0075.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Tulyabaeva, L.I., Salakhutdinov, R.R., Tyumkina, T.V. et al. First Synthesis of a New Class of Spiro-boracarbocycles by Cp2TiCl2-Catalyzed Cycloboration of Methylidenecycloalkanes with PhBCl2. Russ J Org Chem 58, 1902–1908 (2022). https://doi.org/10.1134/S107042802212020X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042802212020X