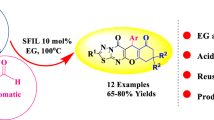
Abstract
An effective and environmentally benign methodology has been proposed for the synthesis of isoxazol-5(4H)-one derivatives using GO@Fe(ClO4)3 nanocatalyst. The one-pot multicomponent reaction of aldehydes, hydroxylamine hydrochloride, and ethyl acetoacetate under solvent-free conditions at 100°C afforded the corresponding 4-(arylmethylidene)-3-methyl-1,2-oxazol-5(4H)-ones in good to excellent yields. The developed new protocol is environmentally friendly as it is characterized by safety, atom efficiency, low cost, mild solvent-free conditions, minimal waste, catalyst recyclability, easy workup, and excellent functional group tolerance for the synthesis of structurally diverse isoxazole derivatives. All products were characterized by spectral and analytical methods. Also, some new compounds were synthesized.







Similar content being viewed by others
REFERENCES
Vitaku, E., Smith, D.T., and Njardarson, J.T., J. Med. Chem., 2014, vol. 57, p. 10257. https://doi.org/10.1021/jm501100b
Zhu, J., Mo, J., Lin, H.Z., Chen, Y., and Sun, H.P., Bioorg. Med. Chem., 2018, vol. 26, p. 3065. https://doi.org/10.1016/j.bmc.2018.05.013
Hu, F. and Szostak, M., Adv. Synth. Catal., 2015, vol. 357, p. 2583. https://doi.org/10.1002/adsc.201500319
De Oliveira Silva, A., McQuade, J., and Szostak, M., Adv. Synth. Catal., 2019, vol. 361, p. 3050. https://doi.org/10.1002/adsc.201900072
Jadhav, S.Y., Shirame, S.P., Kulkarni, S.D., Patil, S.B., Pasale, S.K., and Bhosale, R.B., Bioorg. Med. Chem. Lett., 2013, vol. 23, p. 2575. https://doi.org/10.1016/j.bmcl.2013.02.105
Reddy, K.R., Rao, P.S., Dev, G.J., Poornachandra, Y., Kumar, C.G., Rao, P.S., and Narsaiah, B., Bioorg. Med. Chem. Lett., 2014, vol. 24, p. 1661. https://doi.org/10.1016/j.bmcl.2014.02.069
Breuer, S., Chang, M.W., Yuan, J., and Torbett, B.E., J. Med. Chem., 2012, vol. 55, p. 4968. https://doi.org/10.1021/jm201442t
Kömürcü, Ş.G., Rollas, S., Yilmaz, N., and Çevıkbaş, A., Drug Metab. Drug Interact., 1995, vol. 12, p. 161. https://doi.org/10.1515/dmdi.1995.12.2.161
Kafle, B., Aher, N.G., Khadka, D., Park, H., and Cho, H., Chem. Asian J., 2011, vol. 6, p. 2073. https://doi.org/10.1002/asia.201100154
Demers, J.P., Hageman, W.E., Johnson, S.G., Klaubert, D.H., Look, R.A., and Moore, J.B., Bioorg. Med. Chem. Lett., 1994, vol. 4, p. 2451. https://doi.org/10.1016/s0960-894x(01)80408-x
Panathur, N., Gokhale, N., Dalimba, U., Koushik, P.V., Yogeeswari, P., and Sriram, D., Bioorg. Med. Chem. Lett., 2015, vol. 25, p. 2768. https://doi.org/10.1016/j.bmcl.2015.05.015
Zhang, Y.-Q., Ma, J.-J., Wang, C., Li, J.-C., Zhang, D.-N., Zang, X.-H., and Li, J., Chin. J. Org. Chem., 2008, vol. 28, p. 141.
Vekariya, R.H.; Prajapati, N.P., Patel, K.D., Mayur, K., Vekariya, M.K., Dhaval, B., Patel, D.B., Hitesh, D., and Patel, H.D., World J. Pharm. Pharm. Sci., 2017, vol. 6, p. 2003. https://doi.org/10.20959/wjpps201704-9010
Liu, Q. and Wu, R.-T., J. Chem. Res., Synop., 2011, vol. 35, p. 598. https://doi.org/10.3184/174751911X13176501108975
Kiyani, H. and Ghorbani, F., Res. Chem. Intermed., 2015, vol. 41, p. 2653. https://doi.org/10.1007/s11164-013-1411-x
Setamdideh, D., J. Mex. Chem. Soc., 2015, vol. 59, p. 191. https://doi.org/10.29356/jmcs.v59i3.34
Liu, Q. and Zhang, Y.N., Bull. Korean Chem. Soc., 2011, vol. 32, p. 3559. https://doi.org/10.5012/bkcs.2011.32.10.3559
Khodja, I.A., Boulcina, R., Boumoud, T., Boumoud, B., and Debache, A., Pharma Chem., 2016, vol. 8, p. 97.
Pawar, G.T., Gadecar, S.P., Arbad, B.R., and Lande, M.K., Bull. Chem. React. Eng. Cat., 2017, vol. 12, p. 32. https://doi.org/10.9767/bcrec.12.1.655.32-40
Ghosh, S., Saikh, F., Das, J., and Pramanik, A.K., Tetrahedron Lett., 2013, vol. 54, p. 58. https://doi.org/10.1016/j.tetlet.2012.10.079
Safari, J., Ahmadzadeh, M., and Zarnegar, Z., Catal. Commun., 2016, vol. 86, p. 91. https://doi.org/10.1016/j.catcom.2016.08.018
Fozooni, S., Hosseinzadeh, N.G., Hamidian, H., and Akhgar, M.R., J. Braz. Chem. Soc., 2013, vol. 24, p.1649. https://doi.org/10.5935/0103-5053.20130211
Khandebharad, A.U., Sarda, S.R., Gill, C.H., and Agrawal, B.R., Res. J. Chem. Sci., 2015, vol. 5, p. 27.
Kiyani, H. and Ghorbani, F., Open J. Org. Chem., 2013, vol. 1, p. 5.
Kiyani, H., Org. Chem.: Indian J., 2013, vol. 9, p. 97.
Rikani, A.B. and Setamdideh, D., Orient. J. Chem., 2016, vol. 32, p. 1433. https://doi.org/10.13005/ojc/320317
Kiyani, H. and Ghorbani, F., Heterocycl. Lett., 2013, vol. 3, p. 359.
Behbahani, F.K. and Ziaei, P., 2012, Chin. J. Chem., vol. 30, p. 65. https://doi.org/10.1002/cjoc.201180461
Behbahani, F.K. and Yektanezhad, T., Monatsh. Chem., 2012, vol. 143, p. 1529. https://doi.org/10.1007/s00706-012-0724-6
Moniri, Z. and Behbahani, F.K., Scholars Int. J. Chem. Mater. Sci., 2019, vol. 2, p. 115. https://doi.org/10.36348/sijcms.2019.v02i07.002
Irannejad-Gheshlaghchaei, N., Zare, A., Sajadikhah, S.S., and Banaei, A., Res. Chem. Intermed., 2018, vol. 44, p. 6253. https://doi.org/10.1007/s11164-018-3488-8
Patil, M.S., Mudaliar, C., and Chaturbhuj, G.U., Tetrahedron Lett., 2017, vol. 58, p. 3256. https://doi.org/10.1016/j.tetlet.2017.07.019
Pourmousavi, S.A., Fattahi, H.R., Ghorbani, F., Kanaani, A., and Ajloo, D., J. Iran. Chem. Soc., 2018, vol. 15, p. 455. https://doi.org/10.1007/s13738-017-1246-2
Ahmadzadeh, M., Zarnegar, Z., and Safari, J., Green Chem. Lett. Rev., 2018, vol. 11, p. 78. https://doi.org/10.1080/17518253.2018.1434564
Saikh, F., Das, J., and Ghosh, S., Tetrahedron Lett., 2013, vol. 54, p. 4679. https://doi.org/10.1016/j.tetlet.2013.06.086
Khandebharad Amol, U., Sarda Swapnil, R., Gill Charansingh, H., and Agrawal Brijmohan, R., Res. J. Chem. Sci., 2015, vol. 5, p. 1.
Laroum, R. and Debache, A., Synth. Commun., 2018, vol. 48, p. 1876. https://doi.org/10.1080/00397911.2018.1473440
Wang, Y., Sang, R., Zheng, Y., Guo, L., Guan, M., and Wu, Y., Catal. Commun., 2017, vol. 89, p. 138. https://doi.org/10.1016/j.catcom.2016.09.027
Heravi, M.M. and Behbahani, F.K., J. Iran. Chem. Soc., 2007, vol. 4, p. 375. https://doi.org/10.1007/bf03247223
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Madandar, E., Behbahani, F.K. Green Route for the Synthesis of 3,4-Disubstituted Isoxazol-5(4H)-ones Using GO@Fe(ClO4)3 Nanocatalyst under Solvent-Free Conditions. Russ J Org Chem 58, 830–836 (2022). https://doi.org/10.1134/S1070428022060112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022060112