Abstract
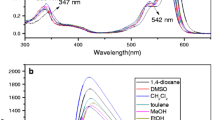
A new and simple colorimetric receptor 1 was prepared easily by one-step condensation of 3-acetyl-4-hydroxycoumarin and 8-aminoquinoline. Its absorbance at λ 296 nm significantly increased upon addition of Fe3+ with a turn-on mode. Furthermore, turn-off sensing happened when F– was added to the 1–Fe3+ complex formed in situ. The 1–Fe3+ complex showed high selectivity and low detection limit toward F– ion. Free Schiff base 1 was then given off for recognition Fe3+ again. The reversible “off-on-off” sensing occurred upon sequential addition of Fe3+ and F–.









Similar content being viewed by others
REFERENCES
Yang, Y., Zhao, Q., Feng, W., and Li, F., Chem. Rev., 2013, vol. 113, p. 192. https://doi.org/10.1021/cr2004103
Dong, X., Zhou, Y., Song, Y., and Qu, J., J. Fluorine Chem., 2015, vol. 178, p. 61. https://doi.org/10.1016/j.jfluchem.2015.06.025
Galaris, D., Skiada, V., and Barbouti, A., Cancer Lett., 2008, vol. 266, p. 21. https://doi.org/10.1016/j.canlet.2008.02.038
Philpott, C.C., J. Biol. Chem., 2012, vol. 287, p. 13518. https://doi.org/10.1074/jbc.R111.326876
Kirk, K.L., Biochemistry of Halogens and Inorganic Halides, New York: Plenum, 1991, p. 58.
Kleerekoper, M., Endocrinol. Metab. Clin. North Am., 1998, vol. 27, p. 441. https://doi.org/10.1016/S0889-8529(05)70015-3
Briancon, D., Rev. Rhum., 1997, vol. 64, p. 78.
Everett, E.T., J. Dent. Res., 2011, vol. 90, p. 552. https://doi.org/10.1177/0022034510384626
Cittanova, M.-L., Lelongt, B., Verpont, M.-C., Géniteau-Legendre, M., Wahbé, F., Prié, D., Coriat, P., and Ronco, P.M., Anesthesiology, 1996, vol. 84, p. 428. https://doi.org/10.1097/00000542-199602000-00022
Ludlow, M., Luxton, G., and Mathew, T., Nephrol. Dial. Transplant., 2007, vol. 22, p. 2763. https://doi.org/10.1093/ndt/gfm477
Singh, P.P., Barjatiya, M.K., Dhing, S., Bhatnagar, R., Kothari, S., and Dhar, V., Urol. Res., 2001, vol. 29, p. 238. https://doi.org/10.1007/s002400100192
Dalapati, S., Alam, M.A., Jana, S., Karmakar, S., and Guchhait, N., Spectrochim. Acta, Part A, 2013, vol. 102, p. 314. https://doi.org/10.1016/j.saa.2012.10.038
Dalapati, S., Alam, M.A., Jana, S., and Guchhait, N., Sens. Actuators, B, 2012, vol. 162, p. 57. https://doi.org/10.1016/j.snb.2011.12.022
Dalapati, S., Jana, S., and Guchhait, N., Chem. Lett., 2011, vol. 40, p. 279. https://doi.org/10.1246/cl.2011.279
Dalapati, S., Jana, S., Alam, M.A., and Guchhait, N., Sens. Actuators, B, 2011, vol. 160, p. 1106. https://doi.org/10.1016/j.snb.2011.09.034
Lavigne, J.J. and Anslyn, E.V., Angew. Chem., Int. Ed., 1999, vol. 38, p. 3666. https://doi.org/10.1002/(SICI)1521-3773(19991216)38:24<3666::AID-ANIE3666>3.0.CO;2-E
Metzger, A. and Anslyn, E.V., Angew. Chem., Int. Ed., 1998, vol. 37, p. 649. https://doi.org/10.1002/(SICI)1521-3773(19980316)37:5<649::AID-ANIE649>3.0.CO;2-H
Goswami, S., Paul, S., and Manna, A., Tetrahedron Lett., 2014, vol. 55, p. 3946. https://doi.org/10.1016/j.tetlet.2014.05.018
Bhalla, V., Arora, H., and Kumar, M., Dalton Trans., 2013, vol. 42, p. 4450. https://doi.org/10.1039/C2DT32427J
Bhalla, V., Singh, H., and Kumar, M., Dalton Trans., 2012, vol. 41, p. 11413. https://doi.org/10.1039/C2DT31244A
Liu, Y., Lv, X., Zhao, Y., Liu, J., Sun, Y.-Q., Wang, P., and Guo, W., J. Mater. Chem., 2012, vol. 22, p. 1747. https://doi.org/10.1039/C1JM15072C
Jung, H.S., Han, J.H., Kim, Z.H., Kang, C., and Kim, J.S., Org. Lett., 2011, vol. 13, p. 5056. https://doi.org/10.1021/ol2018856
Chen, X., Nam, S.-W., Kim, G.-H., Song, N., Jeong, Y., Shin, I., Kim, S.K., Kim, J., Park, S., and Yoon, J., Chem. Commun., 2010, vol. 46, p. 8953. https://doi.org/10.1039/C0CC03398G
Lou, X., Zhang, L., Qin, J., and Li, Z., Chem. Commun., 2008, p. 5848. https://doi.org/10.1039/B812746H
Chung, S.-Y., Nam, S.-W., Lim, J., Park, S., and Yoon, J., Chem. Commun., 2009, p. 2866. https://doi.org/10.1039/B901140D
Gao, G.-Y., Qu, W.-J., Shi, B.-B., Lin, Q., Yao, H., Zhang, Y.-M., Chang, J., Cai, Y., and Wei, T.-B., Sens. Actuators, B, 2015, vol. 213, p. 501. https://doi.org/10.1016/j.snb.2015.02.077
Liu, J., Lin, Q., Zhang, Y.-M., and Wei, T.-B., Sens. Actuators, B, 2014, vol. 196, p. 619. https://doi.org/10.1016/j.snb.2014.02.062
Luxami, V., Paul, K., and Jeong, I.H., Dalton Trans., 2013, vol. 42, p. 3783. https://doi.org/10.1039/C2DT32516K
Jiao, S.-Y., Li, K., Zhang, W., Liu, Y.-H., Huang, Z., and Yu, X.-Q., Dalton Trans., 2015, vol. 44, p. 1358. https://doi.org/10.1039/C4DT03022B
You, Q.-H., Lee, A.W.-M., Chan, W.-H., Zhu, X.-M., and Leung, K.C.-F., Chem. Commun., 2014, vol. 50, p. 6207. https://doi.org/10.1039/C4CC00521J
Kaur, S., Bhalla, V., and Kumar, M., Chem. Commun., 2014, vol. 50, p. 9725. https://doi.org/10.1039/C4CC03877K
Hou, J.-T., Li, K., Yu, K.-K., Wu, M.-Y., and Yu, X.-Q., Org. Biomol. Chem., 2013, vol. 11, p. 717. https://doi.org/10.1039/C2OB26955D
Wang, H., Zhou, G., and Chen, X., Sens. Actuators, B, 2013, vol. 176, p. 698. https://doi.org/10.1016/j.snb.2012.10.006
Shi, Y., Yao, J., Duan, Y., Mi, Q., Chen, J., Xu, Q., Gou, G., Zhou, Y., and Zhang, J., Bioorg. Med. Chem. Lett., 2013, vol. 23, p. 2538. https://doi.org/10.1016/j.bmcl.2013.03.004
Kumar, M., Kumar, R., and Bhalla, V., Tetrahedron Lett., 2010, vol. 51, p. 5559. https://doi.org/10.1016/j.tetlet.2010.08.041
Bhalla, V., Gupta, A., and Kumar, M., Talanta, 2013, vol. 105, p. 152. https://doi.org/10.1016/j.talanta.2012.11.044
Lu, W., Jiang, H., Hu, F., Jiang, L., and Shen, Z., Tetrahedron, 2011, vol. 67, p. 7909. https://doi.org/10.1016/j.tet.2011.08.035
Ghosh, K. and Tarafdar, D., Supramol. Chem., 2015, vol. 27, p. 224. https://doi.org/10.1080/10610278.2014.969264
Liu, J., Xie, Y.-Q., Lin, Q., Shi, B.-B., Zhang, P., Zhang, Y.-M., and Wei, T.-B., Sens. Actuators, B, 2013, vol. 186, p. 657. https://doi.org/10.1016/j.snb.2013.06.080
Dalapati, S., Jana, S., and Guchhait, N., Spectrochim. Acta, Part A, 2014, vol. 129, p. 499. https://doi.org/10.1016/j.saa.2014.03.100
Funding
The authors gratefully acknowledge the financial support of Shandong Provincial Natural Science Foundation (no. ZR2018LB012 and no. ZR2017ZC0529).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, Q., Liu, H. et al. Synthesis and Fluoride Detection Properties of a Coumarin Derivative. Russ J Org Chem 56, 2222–2227 (2020). https://doi.org/10.1134/S1070428020120271
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020120271