Abstract
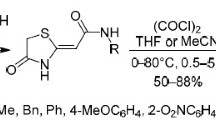
(Z)-3-(2-Aryl-2-oxoethylidene)morpholin-2-ones were synthesized by the reaction of aroylpyruvic acids with ethanolamine or 2-propanolamine. The products reacted with oxalyl chloride to form 8-aroyl-3,4-dihydropyrrolo[2,1-c][1,4]oxazin-1,6,7(1H)-triones.




Similar content being viewed by others
Notes
For preliminary communication, see [18].
REFERENCES
Maslivets, A.N., Mashevskaya, I.V., Krasnykh, O.P., Shurov, S.N., and Andreichikov, Y.S., Zh. Org. Khim., 1992, vol. 28, p. 2545.
Aliev, Z.G., Krasnykh, O.P., Maslivets, A.N., and Atovmyan, L.O., Izv. Akad. Nauk, Ser. Khim., 2000, vol. 12, p. 2080.
Maslivets, A.N., Golovnina, O.V., Krasnykh, O.P., and Aliev, Z.G., Chem. Heterocycl. Compd., 2000, vol. 36, p. 105. https://doi.org/10.1007/BF02256855
Tolmacheva, I.A., Mashevskaya, I.V., and Maslivets, A.N., Russ. J. Org. Chem., 2001, vol. 37, p. 596. https://doi.org/10.1023/A:1012458608681
Mashevskaya, I.V., Makhmudov, R.R., Aleksandrova, G.A., Golovnina, O.V., Duvalov, A.V., and Maslivets, A.N., Khim.-Farm. Zh., 2001, vol. 35, p. 20.
Tolmacheva, I.A., Mashevskaya, I.V., and Maslivets, A.N., Russ. J. Org. Chem., 2002, vol. 38, p. 281. https://doi.org/10.1023/A:1015590306099
Maslivets, A.N., Mashevskaya, I.V., Duvalov, A.V., Kol’tsova, S.V., and Feshin, V.P., Russ. J. Org. Chem., 2002, vol. 38, p. 738. https://doi.org/10.1023/A:1019679526434
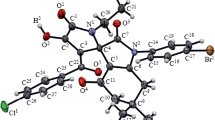
Aliev, Z.G., Maslivets, A.N., Golovnina, O.V., Krasnykh, O.P., Atovmyan, L.O., Zh. Strukt. Khim., 2002, vol. 43, p. 576.
Kistanova, N.S., Mashevskaya, I.V., Bozdyreva, K.S., and Maslivets, A.N., Chem. Heterocycl. Compd., 2003, vol. 39, p. 673. https://doi.org/10.1023/A:1025170821406
Vostrov, E.S., Gilev, D.V., and Maslivets, A.N., Chem. Heterocycl. Compd., 2004, vol. 40, p. 532. https://doi.org/10.1023/B:COHC.0000033556.58356.5c
Bozdyreva, K.S., Smirnova, I.V., and Maslivets, A.N., Russ. J. Org. Chem., 2005, vol. 41, p. 1081. https://doi.org/10.1007/s11178-005-0296-6
Semenova, T.D. and Krasnykh, O.P., Russ. J. Org. Chem., 2005, vol. 41, p. 1222. https://doi.org/10.1007/s11178-005-0321-9
Chervyakov, A.V. and Maslivets, A.N., Russ. J. Org. Chem., 2013, vol. 49, p. 943. https://doi.org/10.1134/S1070428013060286
Maslivets, A.N., Lisovenko, N.Yu., Golovnina, O.V., Vostrov, E.S., and Tarasova, O.P., Chem. Heterocycl. Compd., 2000, vol. 36, p. 483. https://doi.org/10.1007/BF02269553
Silaichev, P.S., Kryuchkova, M.A., and Maslivets, A.N., Russ. J. Org. Chem., 2009, vol. 45, p. 1730. https://doi.org/10.1134/S1070428009110293
Andreichikov, Yu.S., Voronova, L.A., Astaf’eva, I.Yu., Tendryakova, S.V., and Belykh, Z.D., USSR Inventor’s Certificate no. 621676, 1978.
Maslivets, A.N. and Mashevskaya, I.V., 2,3-Digidro-2,3-pirroldiony (2,3-Dihydropyrrol-2,3-diones), Perm: Perm. Gos. Univ., 2005.
Tretyakov, N.A., Shavrina, T.V., and Maslivets, A.N., Russ. J. Org. Chem., 2019, vol. 55, p. 719. https://doi.org/10.1134/S1070428019050221
Allen, F.H., Kennard, O., Watson, D.G., Brammer, L., Orpen, A.G., and Taylor, R., J. Chem. Soc., Perkin Trans. 2, 1987, S1. https://doi.org/10.1039/P298700000S1
CrysAlisPro, Agilent Technologies, Version 1.171.37.33 (release 27-03-2014 CrysAlis171 .NET).
Sheldrick, G.M., Acta Crystallogr. Sect. A., 2008, vol. 64, p. 112. https://doi.org/10.1107/S0108767307043930
Sheldrick, G.M., Acta Crystallogr. Sect. C., 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J, Howard, J.A.K., and Puschmann, H., J. Appl. Cryst., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Funding
The work was financially supported by the Russian Foundation for Basic Research (project no. 19-33-90222) and the Government of the Perm Krai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tret’yakov, N.A., Dmitriev, M.V. & Maslivets, A.N. Synthesis of Pyrrolo[2,1-c][1,4]oxazine-1,6,7-triones by the Reaction of 3-Methylenemorpholin-2-ones with Oxalyl Chloride. Russ J Org Chem 56, 1367–1373 (2020). https://doi.org/10.1134/S1070428020080060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020080060