Abstract
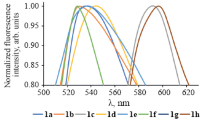
Potassium salts of arylmethylidene derivatives of malononitrile trimer were obtained by the reactions of aromatic aldehydes with malononitrile trimer in an aqueous medium under micellar catalysis. The optical properties of the synthesized salts were studied to show they absorb in the range of 313–436 nm, depending on the substituent. Exchange reactions with various metal salts showed that the synthesized derivatives of malononitrile trimer form difficultly soluble compounds with silver salts.



Similar content being viewed by others
REFERENCES
Dupouy, G., Triki, S., Marchivie, M., Cosquer, N., Gómez-García, C. J., Pillet, S., Bendeif, E.-E., Lecomte, C., Asthana, S., and Létard, J.-F., Inorg. Chem., 2010, vol. 49, p. 9358. https://doi.org/10.1021/ic101038z
Sekizaki, S., Tada, C., Yamochi, H., and Saito, G., J. Mater.Chem., 2001, vol. 11, p. 2293. https://doi.org/10.1039/b105340j
Dupouy, G., Marchivie, M., Triki, S., Sala-Pala, J., Gómez-García, C.-J., Pillet, S., Lecomte, C., and Létard, J.-F., Chem. Comm., 2009, p. 3404. https://doi.org/10.1039/b902339a
Zhao, D., Li, J., Bi, F., Yang, Y., Gao, X., Zhang, W., Zhang, G., and Gao, Z., Z. Naturforsch. B Chem. Sci., 2015, vol. 70, p. 317. https://doi.org/10.1515/znb-2014-0251
Liu, Y., Tang, L.-Z., and Zhan, S.-Z., Inorg. Chem.Commun., 2017, vol. 75, p. 49. https://doi.org/10.1016/j.inoche.2016.12.003
Grigor’ev, A.A., Karpov, S.V., Kayukov, Ya.S., Belikov, M.Yu., and Nasakin, O.E., Tetrahedron Lett., 2015, vol. 56, p. 627. https://doi.org/10.1016/j.tetlet.2015.09.130
Khil, A.M., Kaminskii, V.A., Slabko, O.Yu., Kachanov, A.V., and Gerasimenko, A.V., J. Heterocycl.Chem., 2014, vol. 52, p. 688. https://doi.org/10.1002/jhet.2157
Karpov, S.V., Kayukov, Y.S., Bardasov, I.N., Kayukova, O.V., Ershov, O.V., and Nasakin, O.E., Russ. J. Org.Chem., 2011, vol. 47, p. 405. https://doi.org/10.1134/s1070428011030134
Karpov, S.V., Kayukov, Y.S., Bardasov, I.N., Ershov, O.V., Nasakin, O.E., and Kayukova, O.V., Russ. J. Org.Chem., 2011, vol. 47, p. 1161. https://doi.org/10.1134/s1070428011080070
Karpov, S.V., Kayukov, Y.S., Bardasov, I.N., Kayukova, O.V., Lipin, K.V., and Nasakin, O.E., Russ. J. Org.Chem., 2011, vol. 47, p. 1492. https://doi.org/10.1134/s107042801110006x
Kaminskii, V.A., Slabko, O.Y., Kachanov, A.V., and Buhvetskii, B.V., Tetrahedron Lett., 2003, vol. 44, p. 139. https://doi.org/10.1016/S0040-4039(02)02509-1
Luo, S.-P., Peng, Q.-X., Liu, J., and Zhan, S.-Z., Polyhedron, 2018, vol. 139, p. 44. https://doi.org/10.1016/j.poly.2017.10.010
Middleton, W.J., Little, E.L., Coffman, D.D., and Engelhardt, V.A., J. Am. Chem. Soc., 1958, vol. 80, p. 2795. https://doi.org/10.1021/ja01544a055
Bardasov, I.N., Alekseeva, A.U., Tafeenko, V.A., and Ershov, O.V., Tetrahedron Lett., 2017, vol. 58, p. 4003. https://doi.org/10.1016/j.tetlet.2017.09.012
Kelly, R.B., Slomp, G., and Caron, E.L., J. Org. Chem., 1965, vol. 30, p. 1036. https://doi.org/10.1021/jo01015a020
Vashishtha, M., Mishra, M., and Shah, D.O., GreenChem., 2016, vol. 18, p. 1339. https://doi.org/10.1039/c5gc01966d
Ershov, O.V., Bardasov, I.N., Alekseeva, A.Y., Ievlev, M.Y., and Belikov, M.Y., Russ. J. Org. Chem., 2017, vol. 53, p. 1025. https://doi.org/10.1134/s1070428017070107
Vashishtha, M., Mishra, M., and Shah, D.O., Appl. Catal.Gen., 2013, vol. 466, p. 38. https://doi.org/10.1016/j.apcata.2013.06.015
Bardasov, I.N., Alekseeva, A.Yu., and Ershov, O.V., Russ.J. Org. Chem., 2017, vol. 53, p. 1270. https://doi.org/10.1134/s107042801708019x
Bezgin, D.A., Ershov, O.V., Ievlev, M.Y., Belikov, M.Y., and Bardasov, I.N., Russ. J. Org. Chem., 2018, vol. 54, p. 1100. https://doi.org/10.1134/s1070428018070217
Bardasov, I.N., Alekseeva, A.Y., Bezgin, D.A., and Ershov, O.V., Russ. J. Org. Chem., 2018, vol. 54, p. 1839. https://doi.org/10.1134/s10704280181201875
Funding
The study was funded from a grant of the President of the Russian Federation for state support of young Russian scientists (grant no. MK-2166.2019.3, agreement no. 075-15-2019-383).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Alekseeva, A.U., Dianov, N.P., Yashchenko, N.N. et al. Micelle-Catalyzed Synthesis of Arylmethylidene Derivatives of Malononitrile Trimer and Study of Their Optical Properties. Russ J Org Chem 56, 763–767 (2020). https://doi.org/10.1134/S1070428020050061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020050061