Abstract
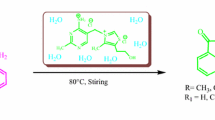
An efficient method has been developed for the synthesis of α,ω-di(spiro[adamantane-2,3′[1,2, 4,5,7]tetroxazocan]-7′-yl)alkanes by recyclization of spiro[adamantane-2,3′-[1,2,4,5,7]pentoxocane] with α,ω-diaminoalkanes in THF in the presence of Sm(NO3)3 · 6 H2O as catalyst.






Similar content being viewed by others
REFERENCES
Makhmudiyarova, N.N., Khatmullina, G.M., Rakhimov, R.Sh., Meshcheryakova, E.S., Ibragimov, A.G., and Dzhemilev, U.M., Tetrahedron, 2016, vol. 72, p. 3277. https://doi.org/10.1016/j.tet.2016.04.055
Tyumkina, T.V., Makhmudiyarova, N.N., Kiyamutdinova, G.M., Meshcheryakova, E.S., Bikmukhametov, K.Sh., Abdullin, M.F., Khalilov, L.M., Ibragimov, A.G., and Dzhemilev, U.M., Tetrahedron, 2018, vol. 74, p. 1749. https://doi.org/10.1016/j.tet.2018.01.045
Makhmudiyarova, N.N., Rakhimov, R.Sh., Tyumkina, T.V., Meshcheryakova, E.S., Ibragimov, A.G., and Dzhemilev, U.M., Russ. J. Org. Chem., 2019, vol. 55, p. 620. https://doi.org/10.1134/S1070428019050075
Makhmudiyarova, N.N., Ishmukhametova, I.R., Tyumkina, T.V., Ibragimov, A.G., and Dzhemilev, U.M., Tetrahedron Lett., 2018, vol. 59, p. 3161. https://doi.org/10.1016/j.tetlet.2018.07.010
Makhmudiyarova, N.N., Ishmukhametova, I.R., Dzhemileva, L.U., Tyumkina, T.V., D’yakonov, V.A., Ibragimov, A.G., and Dzhemilev, U.M., RSC Adv., 2019, vol. 9, p. 18923. https://doi.org/10.1039/C9RA02950H
Zheng, W., Wojtas, L., and Antilla, J.C., Angew. Chem., Int. Ed., 2010, vol. 49, p. 6589. https://doi.org/10.1002/anie.201002972
Blumenthal, H. and Liebscher, J., Arkivoc, 2009, vol. 2009, part (xi), p. 204. https://doi.org/10.3998/ark.5550190.0010.b18
Rebek, J. and McCready, R., J. Am. Chem. Soc., 1980, vol. 102, p. 5602. https://doi.org/10.1021/ja00537a033
Rebek, J., Heterocycles, 1981, vol. 15, p. 517. https://doi.org/10.3987/S-1981-01-0517
Chung, L.W., Hayashi, S., Lundberg, M., Nakatsu, T., Kato, H., and Morokuma, K., J. Am. Chem. Soc., 2008, vol. 130, p. 12880. https://doi.org/10.1021/ja8052464
Ellis, G.L., Amewu, R., Sabbani, S., Stocks, P.A., Shone, A., Stanford, D., Gibbons, P., Davies, J., Vivas, L., Charnand, S., Bongard, E., Hall, C., Rimmer, K., Lozanom, S., Jesus, M., Gargallo, D., Ward, S.A., and O’Neill, P.M., J. Med. Chem., 2008, vol. 51, p. 2170. https://doi.org/10.1021/jm701435h
Opsenica, I., Opsenica, D., Lanteri, C.A., Anova, L., Milhous, W.K., Smith, K.S., and Solaja, B.A., J. Med. Chem., 2008, vol. 51, p. 6216. https://doi.org/10.1021/jm8006905
Rode, A.B., Chung, K., Kim, Y.W., and Hong, I.S., Energy Fuels, 2010, vol. 24, p. 1636. https://doi.org/10.1021/ef9013899
Tulyabaev, A.R., Bikmukhametov, K.S., Mescheryakova, E.S., Makhmudiyarova, N.N., Rakhimov, R.S., and Khalilov, L.M., Cryst. Eng. Commun., 2018, vol. 20, p. 3207. https://doi.org/10.1039/c8ce00481a
Allen, F.H., Kennard, O., Watson, D.G., Brammer, L., Orpen, A.G., and Taylor, R., J. Chem. Soc., Perkin Trans. 2, 1987, p. S1. https://doi.org/10.1039/P298700000S1
Makhmudiyarov, N.N., Khatmullina, G.M., Rakhimov, R.Sh., Ibragimov, A.G., and Dzhemilev, U.M., Arkivoc, 2016, vol. 2016, part (v), p. 427. https://doi.org/10.24820/ark.5550190.p009.565
CrysAlis PRO, Yarnton, Oxfordshire, England: Agilent Technologies, 2012.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., and Puschmann, H., J. Appl. Crystallogr., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Sheldrick, G.M., Acta Crystallogr., Sect. A, 2008, vol. 64, p. 112. https://doi.org/10.1107/S0108767307043930
Macrae, C.F., Edgington, P.R., McCabe, P., Pidcock, E., Shields, G.P., Taylor, R., Towler, M., and van de Streek, J., J. Appl. Crystallogr., 2006, vol. 39, p. 453. https://doi.org/10.1107/S002188980600731X
Funding
This study was performed under financial support by the Russian Science Foundation (project no. 18-73-00 014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Makhmudiyarova, N.N., Koroleva, L.S., Meshcheryakova, E.S. et al. Efficient Catalytic Synthesis of α,ω-Di(spiro[adamantane2,3′-[1,2,4,5,7]tetroxazocan]-7′-yl)alkanes. Russ J Org Chem 56, 378–384 (2020). https://doi.org/10.1134/S1070428020030021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020030021