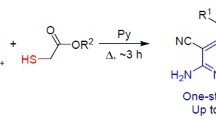
Abstract
Reactions of 1,3-diaryl(heteryl)prop-2-ene-1-thiones with 2-cyanoethanethio(seleno)-amides and alkyl halides led to the formation of substituted 2-alkylsulfanyl-4,6-diaryl(heteryl)-1,4-dihydropyridine-, pyridine-, and thieno[2,3-b]pyridine-3-carbonitriles.
Similar content being viewed by others
References
Tasaka, S., Kine, A., Omori, H., Tanabe, Y., and Gomi, N., EP Patent Appl. no. 1055672, 2000; Chem. Abstr., 2001, no. 19O117P.
Burke, P.J. and Kuox, R.J., GB Patent Appl. no. 2365338, 2002; Ref. Zh. Khim., 2002, no. 19O62P.
Tirzite, D., Krauze, A., Zubareva, A., Tirzitis, G., and Duburs, G., Chem. Heterocycl. Compd., 2002, vol. 38, p. 795.
Sirisha, K., Bikshapathi, D., Achaiah, G., and Reddy, V.M., Eur. J. Med. Chem., 2011, vol. 46, p. 1564.
Gein, V.L., Kazantseva, M.I., Kurbatova, A.A., and Voronina, E.V., Pharm. Chem. J., 2011, vol. 45, p. 474.
Nakajo, A., Tokumasu, M., Kito, M., Takahara, A., Ono, Y., Takeda, T., Kajiagava, Y., and Kaganei, H., US Patent no. 6610717, 2003; Ref. Zh. Khim., 2004, no. 19O83P.
Jacobson, K.A. and Li, A.-H., US Patent no. 6376521, 2002; Ref. Zh. Khim., 2003, no. 19O87P.
Krauze, A., Baumane, L., Sile, L., Chernova, L., Vilums, M., Vitolina, R., Duburs, G., and Stradins, J., Chem. Heterocycl. Compd., 2004, vol. 40, p. 876.
Barfacker, L., Kolkhof, P., Schlemmer, K.-H., Grosser, R., and Nitsche, A., German Patent Appl. no. 102006044696, 2008; Ref. Zh. Khim., 2009, no. 19O121P.
Osolodkin, D.I., Kozlovskaya, L.I., Dueva, E.V., Dotsenko, V.V., Rogova, Y.V., Frolov, K.A., Krivokolysko, S.G., Romanova, E.G., Morozov, A.S., Karganova, G.G., Palyulin, V.A., Pentovski, V.M., and Zefirov, N.S., Med. Chem. Lett., 2013, vol. 4, p. 869.
Nakujo, A., Tokumasu, M., Kito, M., Takahara, A., Ono, Y., Takeda, T., Kajigaya, Y., and Koganei, H., EP Patent Appl., no. 1191022, 2002; Ref. Zh. Khim., 2002, no. 19O69P.
Niwa, S., Ohno, S., Takahara, A., and Kito, M., EP Patent Appl., no. 1123923, 2001; Ref. Zh. Khim., 2002, no. 19O112P.
Ertan, R., Ayhan-Kilcigil, G., and Tunobilek, M., Turk. Bull. Hyg. Experim. Biol., 1998, vol. 55, p. 55; Ref. Zh. Khim., 2000, no. 06-19Zh218.
Litvinov, V.P., Krivokolysko, S.G., and Dyachenko, V.D., Chem. Heterocycl. Compd., 1999, vol. 35, p. 509.
Livinov, V.P., Russ. Chem. Rev., 2006, vol. 75, p. 577.
Eisnen, U. and Kuthan, I., Chem. Rev., 1972, vol. 72, p. 1.
Litvinov, V.P., Sharanin, Yu.A., Shestopalov, A.M., and Dyachenko, V.D., Syntett, 1992, p. 87.
Attia, A.M. and Elgemeie, G.H., Synth. Commun., 2003, vol. 33, p. 2243.
Leistner, S., Ludwig, A., Reichelf, C., and Schulze, A., EP Patent Appl. no. 1681292, 2006; Ref. Zh. Khim., 2007, no. 19O116P.
Cywin, C.L., Chen, Z., Fleck, R.W., Hao, M.-H., Hickey, E., Liu, W., Marshall, D.R., Nemoto, P., Sorcek, R.J., Sun, S., Wu, J.-P., Morwick, T., and Emeigh, J., US Patent no. 6964956, 2005; Ref. Zh. Khim., 2006, no. 19O69P.
Reichelf, C., Ludwig, A., and Leistner, S., EP Patent Appl. no. 1683799, 2006; Ref. Zh. Khim., 2007, no. 19O115P.
Vatsuro, K.V. and Mishchenko, G.L., Imennye reaktsii v organicheskoi khimii, Moscow: Khimiya, 1976.
Itogi nauki i tekhniki. Organicheskaya khimiya, Moscow: VINITI, 1990, vol. 16.
Sharanin, Yu.A., Dyachenko, V.D., Litvinov, V.P., and Turov, A.V., Zh. Obshch. Khim., 1991, vol. 61, p. 942.
Litvinov, V.P., Rodinovskaya, L.A., Sharanin, Yu.A., Shestopalov, A.M., and Senning, A., Sulfur Rep., 1992, vol. 13, p. 1.
Sharanin, Yu.A., Krivokolysko, S.G., and Dyachenko, V.D., Zh. Org. Khim., 1994, vol. 30, p. 581.
Dyachenko, V.D., Russ. J. Gen. Chem., 2005, vol. 75, p. 440.
Dyachenko, V.D., Russ. J. Gen. Chem., 2005, vol. 75, p. 447.
Pretsch, E., Buhlmann, P., and Affolter, C., Structure Determination of Organic Compounds, Tables of Spectra Data, Berlin: Springer, 2000.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.V. Dyachenko, V.D. Dyachenko, 2015, published in Zhurnal Organicheskoi Khimii, 2015, Vol. 51, No. 5, pp. 650–655.
Rights and permissions
About this article
Cite this article
Dyachenko, I.V., Dyachenko, V.D. New synthetic approach to substituted 2-alkylsulfanyl-4,6-diaryl(heteryl)-1,4-dihydropyridine-, pyridine-, and thieno[2,3-b]pyridine-3-carbonitriles. Russ J Org Chem 51, 629–635 (2015). https://doi.org/10.1134/S1070428015050073
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428015050073