Abstract
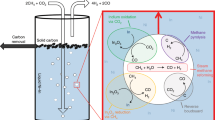
Published data on noncatalytic pyrolysis of natural gas in molten metals are analyzed. The most illustrative results obtained in the past two decades are described. The use of molten metals as reaction medium allows solving the problem of coking of pyrolysis reactors owing to the flotation of the carbon formed to the molten metal surface. The use of liquid metal bubbling reactors allowing the process to be performed at temperatures of up to 1200°С is considered. The maximal conversion was 78% at 1175°С and feeding rate of 50 mL min–1. The major factors favoring more complete conversion of natural gas in the processes under consideration are elevated temperature, decreased gas bubble size due to the use of bubbling systems of various types, and longer residence time of the gas in the heat carrier due to an increase in the reactor length or to use of various types of packing.
Similar content being viewed by others
REFERENCES
Muradov, N., Int. J. Hydrogen Energy, 2017, vol. 42, no. 20, pp. 14058–14088. https://doi.org/10.1016/j.ijhydene.2017.04.101
Catalan, L.J.J. and Rezaei, E., Int. J. Hydrogen Energy, 2020, vol. 45, no. 4, pp. 2486–2503. https://doi.org/10.1016/j.ijhydene.2019.11.143
Mitrova, T., Mel’nikov, Yu., Chugunov, D., and Glagoleva, A., Vodorodnaya ekonomika – put’ to nizkouglerodnomu razvitiyu (Hydrogen Economy—A Way to Low-Carbon Development), Moscow: Skolkovo, 2019.
Muradov, N.Z. and Veziroǧlu, T.N., Int. J. Hydrogen Energy, 2005, vol. 30, no. 3, pp. 225–237. https://doi.org/10.1016/j.ijhydene.2004.03.033
Halloran, J.W., Int. J. Hydrogen Energy, 2008, vol. 33, no. 9, pp. 2218–2224. https://doi.org/10.1016/j.ijhydene.2008.02.074
Muradov, N.Z., Int. J. Hydrogen Energy, 1993, vol. 18, no. 3, pp. 211–215. https://doi.org/10.1016/0360-3199(93)90021-2
Trottier, D., Flynn, M.R., Kostiuk, L., and Secanell, M., Int. J. Hydrogen Energy, 2017, vol. 42, no. 40, pp. 25166–25184. https://doi.org/10.1016/j.ijhydene.2017.08.134
Amin, A.M., Croiset, E., Amin, A.M., and Epling, W., Int. J. Hydrogen Energy, 2011, vol. 36, no. 4, pp. 2904–2935. https://doi.org/10.1016/j.ijhydene.2010.11.035
Abbas, H.F. and Wan Daud, W.M.A., Int. J. Hydrogen Energy, 2010, vol. 35, no. 3, pp. 1160–1190. https://doi.org/10.1016/j.ijhydene.2009.11.036
Yan, W. and Hoekman, S.K., Environ. Prog. Sustain. Energy, 2014, vol. 33, no. 1, pp. 213–219. https://doi.org/10.1002/ep.11746
Abánades, A., Ruiz, E., Ferruelo, E.M., Hernández, F., Cabanillas, A., Martínez-Val, J.M., Rubio, J.A., López, C., Gavela, R., Barrera, G., Rubbia, C., Salmieri, D., Rodilla, E., and Gutiérrez, D., Int. J. Hydrogen Energy, 2011, vol. 36, no. 20, pp. 12877–12886. https://doi.org/10.1016/j.ijhydene.2011.07.081
Patent US 1803221 A, Publ. 1931
Steinberg, M., Energy Convers. Manag., 1996, vol. 37, no. 6, pp. 843–848. https://doi.org/10.1016/0196-8904(95)00266-9
Steinberg, M., Int. J. Hydrogen Energy, 1999, vol. 24, no. 8, pp. 771–777. https://doi.org/10.1016/S0360-3199(98)00128-1
Senchenko, V.N. and Belikov, R.S., J. Phys.: Conf. Ser., 2017, vol. 891, p. 12338. https://doi.org/10.1088/1742-6596/891/1/01233
Assael, M.J., Kalyva, A.E., Antoniadis, K.D., Michael Banish, R., Egry, I., Wu, J., Kaschnitz, E., and Wakeham, W.A., J. Phys. Chem. Ref. Data, 2010, vol. 39, no. 3, p. 33105. https://doi.org/10.1063/1.3467496
Abánades, A., Mehravaran, K., Rathnam, R.K., Rubbia, C., Salmieri, D., Stoppel, L., Stückrad, S., and Wetzel, Th., Int. J. Hydrogen Energy, 2015, vol. 40, no. 25, pp. 8020–8033. https://doi.org/10.1016/j.ijhydene.2015.04.062
Domínguez, A., Fidalgo, B., Fernández, Y., Pis, J.J., and Menéndez, J.A., Int. J. Hydrogen Energy, 2007, vol. 32, no. 18, pp. 4792–4799. https://doi.org/10.1016/j.ijhydene.2007.07.041
Fidalgo, B., Fernández, Y., Domínguez, A., Pis, J.J., and Menéndez, J.A., J. Anal. Appl. Pyrol., 2008, vol. 82, no. 1, pp. 158–162. https://doi.org/10.1016/j.jaap.2008.03.004
Chen, W.-H., Liou, H.-J., and Hung, Ch.-I., Int. J. Hydrogen Energy, 2013, vol. 38, no. 30, pp. 13260–13271. https://doi.org/10.1016/j.ijhydene.2013.07.107
Fulcheri, L. and Schwob, Y., Int. J. Hydrogen Energy, 1995, vol. 20, no. 3, pp. 197–202. https://doi.org/10.1016/0360-3199(94)E0022-Q
Fulcheri, L., Probst, N., Flamant, G., Fabry, F., Grivei, E., and Bourrat, X., Carbon, 2002, vol. 40, no. 2, pp. 169–176. https://doi.org/10.1016/S0008-6223(01)00169-5
Muradov, N., Smith, F., Bockerman, G., and Scammon, K., Appl. Catal. A, 2009, vol. 365, no. 2, pp. 292–300. https://doi.org/10.1016/j.apcata.2009.06.031
Tsai, C.-H. and Chen, K.-T., Int. J. Hydrogen Energy, 2009, vol. 34, no. 2, pp. 833–838. https://doi.org/10.1016/j.ijhydene.2008.10.061
Dors, M., Nowakowska, H., Jasiński, M., and Mizeraczyk, J., Plasma Chem. Plasma Process., 2014, vol. 34, no. 2, pp. 313–326. https://doi.org/10.1007/s11090-013-9510-4
Patent US 5767165 A, Publ. 1998
Rodat, S., Abanades, S., Coulié, J., and Flamant, G., Chem. Eng. J., 2009, vol. 146, no. 1, pp. 120–127. https://doi.org/10.1016/j.cej.2008.09.008
Serban, M., Lewis, M.A., Marshall, C.L., and Doctor, R.D., Energy Fuels, 2003, vol. 17, no. 3, pp. 705–713. https://doi.org/10.1021/ef020271q
Geißler, T., Plevan, M., Abánades, A., Heinzel, A., Mehravaran, K., Rathnam, R.K., Rubbia, C., Salmieri, D., Stoppel, L., Stückrad, S., Weisenburger, A.б Wenninger, H., and Wetzel, Th., Int. J. Hydrogen Energy, 2015, vol. 40, no. 41, pp. 14134–14146. https://doi.org/10.1016/j.ijhydene.2015.08.102
Geißler, T., Abánades, A., Heinzel, A., Mehravaran, K., Müller, G., Rathnam, R.K., Rubbia, C., Salmieri, D., Stoppel, L., Stückrad, S., Weisenburger, A., Wenninger, H., and Wetzel, Th., Chem. Eng. J., 2016, vol. 299, pp. 192–200. https://doi.org/10.1016/j.cej.2016.04.066
Upham, D.C., Agarwal, V., Khechfe, A., Snodgrass, Z.R., Gordon, M.J., Metiu, H., and McFarland, E.W., Science, 2017, vol. 358, no. 6365, p. 917. https://doi.org/10.1126/science.aao5023
Kozlov, G.I. and Knorre, V.G., Combust. Flame, 1962, vol. 6, pp. 253–263. https://doi.org/10.1016/0010-2180(62)90103-7
Kevorkian, V., Heath, C.E., and Boudart, M., J. Phys. Chem., 1960, vol. 64, no. 8, pp. 964–968. https://doi.org/10.1021/j100837a002
Chen, C.J., Back, M.H., and Back, R.A., Can. J. Chem., 1975, vol. 53, no. 23, pp. 3580–3590.
Martynov, P.N., Gulevich, A.V., Orlov, Yu.I., and Gulevsky, V.A., Prog. Nucl. Energy, 2005, vol. 47, no. 1, pp. 604–615. https://doi.org/10.1016/j.pnucene.2005.05.063
Gulevich, A.V., Martynov, P.N., Gulevsky, V.A., and Ulyanov, V.V., Energy Conv. Manag., 2008, vol. 49, no. 7, pp. 1946–1950. https://doi.org/10.1016/j.enconman.2007.12.028
Paxman, D., Trottier, S., Nikoo, M., Secanell, M., and Ordorica-Garcia, G., Energy Procedia, 2014, vol. 49, pp. 2027–2036. https://doi.org/10.1016/j.egypro.2014.03.215
Schultz, I. and Agar, D.W., Int. J. Hydrogen Energy, 2015, vol. 40, no. 35, pp. 11422–11427. https://doi.org/10.1016/j.ijhydene.2015.03.126
Wang, K., Li, W.S., and Zhou, X.P., J. Mol., 2008, vol. 283, no. 1, pp. 153–157. https://doi.org/10.1016/j.molcata.2007.12.018
Funding
The study was financially supported by the Ministry of Education and Science of the Russian Federation within the framework of agreement no. 05.607.21.0311 оf December 2, 2019, unique agreement identifier RFMEFI60719X0311.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
A.L. Maksimov is the Editor-in-Chief of Zhurnal Prikladnoi Khimii/Russian Journal of Applied Chemistry. The other authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Parfenov, V.E., Nikitchenko, N.V., Pimenov, A.A. et al. Methane Pyrolysis for Hydrogen Production: Specific Features of Using Molten Metals. Russ J Appl Chem 93, 625–632 (2020). https://doi.org/10.1134/S1070427220050018
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220050018