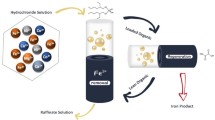
Abstract
The extraction of iron(III) with tributyl phosphate from bromide aqueous solutions in the electrochemical processing of natural waters and brines was studied. It was found that iron(III) is extracted from solutions of alkali metal bromides in the form of the complex salt MFeBr4 at a salt concentration of more than 4 M. Extraction increases in the series KFeBr4 < NaFeBr4 < LiFeBr4. Extraction products are largely susceptible to electrolytic dissociation in the organic phase. The optimal conditions for the extraction of iron(III) bromide and its return to the technological process are determined.





Similar content being viewed by others
REFERENCES
Ksenzenko, V.I. and Stasinevich, D.S., Khimiya i tekhnologiya broma, ioda i ikh soedinenii (Chemistry and Technology of Bromine, Iodine and Their Compounds), Moscow: Khimiya, 1995.
WO Patent 2016054874 (publ. 2016). Method for Extracting Bbromine from Sseawater by Vacuum Distillation.
Liu, W., Cai, R., Zhang, H., Ma, L., Wu, D., Lu, S., and Zhang, Yu-shan, J. Salt Chem. Ind., 2012, no. 5, pp. 20–24
CN Patent 103754823 A (publ. 2014). Reaction-Extraction Coupling Method for Extracting Molecular Bromine or Iodine.
CN Patent 103613072 A (publ. 2014). Sposob izvlecheniya broma iz prirodnykh vod s polucheniem bromidov metallov (Method for Recovering Bromine from Bromine-Containing Wastewater by Solvent Extraction).
Hao, F., Wu, C., Lv, X., Wang, G., and Zhang, H., J. Salt Chem. Ind., 2008, no. 6, pp. 1–4
Fei, G., Yuting, L., Xiushen, Y., and Haining, L., Int. Symp. on Energy Science and Chemical Engineering, 2010, pp. 23–27. https://doi.org/10.2991/isesce-15.2015.5
RF Patent 2360039 (opubl. 2009). Sposob izvlecheniya broma iz prirodnykh khloridnykh vod s polucheniem bromidnogo kontsentrata (The method of Extraction of Bromine from Natural Chloride Waters to Obtain Bromide Concentrate).
RF Patent 2398734 (opubl. 2010). The Method of Extraction of Bromine from Natural Waters to Produce Metal Bromides.
Meyers, D.A. and McDonald, R.L., J. Am. Chem. Soc., 1967, no. 3, pp. 486–489. https://doi.org/10.1021/ja00979a003
Seung, L., Gwang-Seop, L., and Keun Yong, S., Japan Inst. Metals, 2004, vol. 45, no. 6, pp. 1859–1863. https://doi.org/10.2320/matertrans.45.1859
Erickson, R.L. and McDonald, R.L., J. Am. Chem. Soc., 1966, no. 10, pp. 2099–2104. https://doi.org/10.1021/ja00962a003
Gerald, G.S., Herbert, M.P., J. Phys. Chem., 1961, vol. 65, pp. 1932–1935. https://doi.org/10.1021/j100828a004
Kuz’min, V.I., Kuz’mina, A.A., and Gudkova, N.V., Khim. Tekhnologiya, 2018, no. 10, vol. 19, pp. 468–473. https://doi.org/10.31044/1684-5811-2018-19-10-468-473
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
FUNDING
The work was carried out according to the state budget project V.46.1.1 of basic research of the Siberian branch of the Russian Academy of Sciences “Physicochemical studies of the surface and interfacial processes, the development of the scientific foundations of highly effective and environmentally friendly technologies for processing natural and technogenic raw materials of non-ferrous, rare and noble metals and for the production of high-tech materials.”
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kuz’min, D.V., Kuz’min, V.I., Gudkova, N.V. et al. Extraction of Iron(III) with Tributyl Phosphate from Bromide Solutions. Russ J Appl Chem 93, 238–243 (2020). https://doi.org/10.1134/S1070427220020123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220020123