Abstract
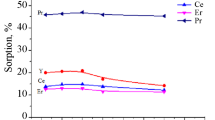
The feasibility of using a macroporous strongly acidic cation exchange resin (SQS-6) as an adsorbent for lanthanum(III) and neodymium(III) from phosphoric acid medium, >4.0 M, was administered using batch and column techniques. Different parameters affecting the sorption of these metal ions such as v/m ratio, acid concentration and the metal ion concentration were separately investigated. The results indicated that the sorption process is relatively fast, reaching equilibrium state within 10 min. Influence of temperature on the equilibrium distribution values was also studied to evaluate the changes in standard thermodynamic quantities where the results indicated that the sorption is endothermic and the process is spontaneous associated with increasing the randomness of the system. The adsorption results of the studied metal ions were found to obey Langmuir isotherm model over the entire studied concentration range. The recovery of La(III) and Nd(III) from the loaded resin was performed with 1.0 M citric acid at pH 4.0. The breakthrough capacity of La(III) and Nd(III) was found to be 33.55 and 17.30 mg/g, respectively. The experimental data resulting from column technique were followed Thomas and Yoon-Nelson models.
Similar content being viewed by others
Change history
19 February 2020
In the head of Table 7, column 3 row 2, Ln(III) should be replaced by Nd(III).
References
Rim, K.T., Koo, K.H., and Park, J.S., Saf. Health Work, 2013, vol. 4, pp. 12–26.
Jarvis, I., Burnett, W.C., Nathan, Y., Almbaydin, S.M.F., Attia, A.K.M., Castro, L.N., Flicoteaux, R., Hilmy, M.E., Husain, V., Qutawah, A.A., Serjani, A., and Zanin, Y.N., In Concepts and Controversies in Phosphogenesis, Eclogae Geol. Helv., 1994, vol. 87, pp. 643–700.
Gupta, C.K., and Krishna, M.N., Extractive Metallurgy of Rare–Earths, London: CRC Press, 2004.
Emsbo, P., McLaughlin, P.A., Breit, G.N., du Bray, E.A., and Koenig, A.E., Gondwana Res., 2015, vol. 27(2), pp. 776–785.
Shengxi, W., Wang, L., Zhao, L., Zhang, P., El–Shall, H., Moudgil, B., Huang, X., and Zhang, L., Chem. Eng. J., 2018, vol. 335, pp. 774–800.
Cherkasov, R.A., Garifzyanov, A.R., Bazanova, E.B., Davletshin, R.R., and Leont’eva, S.V., Russ. J. Gen. Chem., 2012, vol. 82(1), pp. 33–42.
Xie, F., Zhang, T.A., Dreisinger, D., and Doyle, F., Miner. Eng., 2014, vol. 56, pp. 10–28.
Crock, J.G., Lichte, F.E., and Wildeman, T.R., Chem. Geol., 1984, vol. 45(1-2), pp. 149–163.
Kopyrin, A.A., Afonin, M.A., Fomichev, A.A., and Bakharev, M.S., Radiochemistry, 2008, vol. 50(3), pp. 281–285.
Chitra, K.R., Gaikwad, A.G., Surender, G.D., and Damodaran, A.D., J. Membrane Sci., 1997, vol. 125, pp. 257–268.
González, C.H., Cabezas, A.J.Q., and Díaz, M.F., Talanta, 2005, vol. 68(1), pp. 47–53.
Ignatova, S.N., Maryutina, T.A., Spivakov, B.Y., and Karandashev, V.K., Fresen. J. Anal. Chem, 2001, vol. 370(8), pp. 1109–1113.
Kim, I., Kim, S., and Kim, G., Aquat. Geochem., 2010, vol. 16(04), pp. 611–620.
Zhu, Y., Itoh, A., Fujimori, E., Umemura, T., and Haraguchi, H., J. Alloy. Compd., 2006, vol. 408-412, pp. 985–988.
An, F., Gao, B., Huang, X., Zhang, Y., Li, Y., Xu, Y., Zhang, Z., Gao, J., and Chen, Z., J. React. Funct. Polym., 2013, vol. 73(1), pp. 60–65.
Li, X., and Sun, Y., Hydrometallurgy, 2007, vol. 87(1-2), pp. 63–71.
Pradip and Fuerstenau, D.W.F., Int. J. Miner. Process., 1991, vol. 32(1-2), pp. 1–22.
Chirkst, D.E., Lobacheva, O.L., and Dzhevaga, N.V., Russ. J. Appl. Chem., 2011, vol. 84(9), pp.1476–1482.
Tsuruta, T., J. Rare Earth., 2007, vol. 25(5), pp. 526–532.
Chua, H., Sci. Total Environ., 1998, vol. 214(1-3), pp. 79–85.
Beker, P., Hignett, T.P., and Palgrave, D.A., Phosphates and Phosphoric Acid, Raw Materials, Technology and Economics of Wet Process, 2nd ed., New York: Marcel Decker Inc., 1989.
22. Word Fertilizers Trends and Outlook to 2018, Rom: Food and Agriculture Organization of the United Nations, 2015, http://www.fao.org/3/a-i4324e.pdf.
El-Didamony, H., Gado, H.S, Awwad, N.S., Fawzy, M.M., and Attallah, M.F., J. Radioanal. Nucl. Chem., 2012, vol. 291, pp. 907–914.
Jin, H.X., Wu, F.Z., Mao, X.H., Wang, M.L., and Xie, H.Y., Rare Metals, 2017, vol. 36, pp. 840–850.
Reddy, B.R., Kumar, B.N., and Radhika, S., Solvent Extr. Ion Exc., 2009, vol. 27, pp. 695–711.
Kumar, B.N., Radhika, S., Kantam, M.L., and Reddy, B.R., J. Chem Technol. Biotechnol., 2011, vol. 86(4), pp. 562–569.
Reddy, B.R., and Kumar, J.R., Solvent Extr. Ion Exc., 2016, vol. 34, pp. 226–240.
Radhika, S., Nagaraju, V., Kumar, B.N., Kantam, M.L. and Reddy, B.R., J. Rare Earth., 2012, vol. 30, pp. 1270–1275.
Hérès, X., Blet, V., Natale, P.D., Ouaattou, A., Mazouz, H., Dhiba, D., and Cuer, F., Metals, 2018, vol. 8(9), p. 682.
Papkovaa, M.V., Kon’kovab, T.V., Samieva, D.A., and Vasilenko, S.A., Russ. J. Appl. Chem., 2018, vol. 91(3), pp. 379–383.
Kumar, B.N., Radhika, S., and Reddy, B.R. Chem. Eng. J., 2010, vol. 160, pp. 138–144.
Al-Thyabat, S. and Zhang, P., Min. Proc. Ext. Met., 2015, vol. 124, pp. 143–150.
Ismail, I., Ibrahim, M., Aly, H.F., Nomura M., and Fujii, Y., J. Chromatogr. A, 2011, vol. 1218, pp. 2923–2928.
Marczenko, Z., Spectrophotometric Determination of Elements, New York: John Wiley, 1986.
Ahmad, A.A. and Hameed, B., J. Hazard. Mater., 2010, vol. 175, pp. 298–303.
Kundu, S., Kavalakatt, S.S., Pal, A., Ghosh, S.K., Mandal, M., and Pal, T., Water Res., 2004, vol. 38, pp. 3780–3790.
Uddin, Md.T., Rukanuzzaman, M.d., Khan, Md.M.R., and Islam, Md.A., J. Environ. Manage, 2009, vol. 90(11), pp. 3443–3450.
Han, R.P., Zou, L.N., Zhao, X., Xu, Y.F., Xu, F., Li, Y.L., and Wang, Y., Chem. Eng. J., 2009, vol. 149, pp. 123–131.
El–Gammal, B., and Shady, S.A., Colloid Surf. A: Physicochem. Eng. Asp., 2006, vol. 287, pp. 132–138.
Oguz, E., and Ersoy, M., Chem. Eng. J., 2010, vol. 164, pp. 56–62.
Hamed, M.M., J. Radioanal. Nucl. Chem., 2014, vol. 302, pp. 303–313.
Rizk, S.E., and Hamed, M.M., Desalin Water Treat., 2015, vol. 56, pp. 1536–1546.
Hamed, M.M., Ahmed, I.M., and Metwally, S.S., J. Ind. Eng. Chem., 2014, vol. 20, pp. 2370–2377.
Freundlich, H., Colloid and Capillary Chemistry, London: Methuen and Co. Ltd., 1926.
Langmuir, I., J. Am. Chem. Soc., 1918, vol. 40(9), pp. 1361–1403.
Dubinin, M.M., and Radushkevich, L.V., Proc. Acad. Sci. USSR Phys. Chem. Sect., 1947, vol. 55, pp. 331–333.
Huber, H., Stoeckli, H.F., and Houriet, J.P., J. Colloid Interface Sci., 1978, vol. 67, pp. 195–203.
Thomas, H.C., J. Am. Chem. Soc., 1944, vol. 66, pp. 1664–1666.
Yoon, Y.H. and Nelson, J.H., Am. Ind. Hyg. Assoc. J., 1984, vol. 45, pp. 509–516.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu Elgoud, E.M., Ismail, Z.H., Ahmad, M.I. et al. Sorption of Lanthanum(III) and Neodymium(III) from Concentrated Phosphoric Acid by Strongly Acidic Cation Exchange Resin (SQS-6). Russ J Appl Chem 92, 1581–1592 (2019). https://doi.org/10.1134/S1070427219110156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427219110156