Abstract
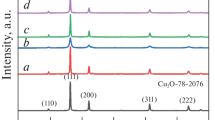
In the present investigation, we synthesized copper (I) oxide nanoparticles (NPs) by the coprecipitation method. The obtained materials were characterized by X-ray diffraction (XRD), energy-dispersive X-ray analysis (EDXA), field emission scanning electron microscopy (FESEM), Transmission Electron Microscopy (TEM), and the Brunauer-Emmett-Teller (BET)/Barrett-Joyner-Halenda (BJH) Method. Surface areas and the average particle size were evaluated to be around 4.20 ± 0.04 m2 g−1 and 28 nm, respectively. Then, Ag/Cu2O NPs were synthesized by the same process, examined by X-ray diffraction, and the average particle size obtained was around 118 nm. The photocatalytic degradation of [1,3-Amino phenyl [4-Sulphonic acid][2,6-Dis azo phenyl] 4,4′sulphato ethyl [6′sulpho] ester of Sulphonic acid] (COG-423) was investigated with Cu2O and TiO2 NPs, Cu2O Microparticles (Micro-Ps) and Ag/Cu2O NPs under UV-C irradiation in the presence of hydrogen peroxide as auxiliary oxidant with three parameters including dopant concentration, intensity, and time, as the obtained experimental results showed a good agreement with theoretical values and succeeded to calculate the optimal conditions. Degradation efficiency with Cu2O Micro/NPs under UV-C irradiation (32 W), for 30 min. were determined to be 20.0% and 91.4% respectively, while for the synthesized TiO2 and Ag/Cu2O, NPs were 99.9%. The photocatalytic activity order was of the following nature: Ag/Cu2O ∼ TiO2 NPs > Cu2O NPs > Cu2O Micro-Ps.
Similar content being viewed by others
References
Elmi Fard, N., Fazaeli, R., and Ghiasi, R., J. Chem. Eng. Tech., 2016, vol. 39, no. 1, pp.149–157.
Kianfar, A.H. and Dostani, M., Russ. J. Appl. Chem., 2017, vol. 28, no. 10, pp. 7353–7359.
Wang, Q., Li, J., Bai, Y., et al., J. Photochem. Photobiol., 2013, vol. 126, pp. 47–54.
Basha, C.A., Sendhil, J., Selvakumar, K.V., et al., Desalin., 2012, vol. 285, pp. 188–197.
Moussavi, G. and Mahmoudi, M., J. Hazard. Mater., 2009, vol. 168, nos. 2–3, pp. 806–812.
Zhang, L., Yan, F., Su, M., et al., Russ. J. Inorg. Chem., 2009, vol. 54, no. 8, pp. 1210–1216.
Liang, Sh., Zhou, Y., Wu, W., and Zheng, Y., J. Photochem. Photobiol. A: Chemistry, 2017, vol. 346, pp. 168–176.
Fujishima, A., Rao, T.N., and Tryk, D.A., J. Photochem. Photobiol. C: Photochemistry Reviews, 2000, vol. 1, no. 1, pp. 1–21.
Fujishima, A., Zhang, X., and Tryk, D. A., Surf. Sci. Rep., 2008, vol. 63, p. 515.
Tseng, C.C., Hsieh, J.H., and Wu, W., Thin Solid Films, 2011, vol. 519, no. 15, pp. 5169–5173.
Bender, M., Seeling, W., Daube, C., et al., J. Stollenwerk, Thin Solid Films, 1998, vol. 326, nos. 1–2, pp. 67–71.
Yu, Y., D, F., Yu, J.C., et al., J. Solid State Chem., 2004, vol. 177, no. 12, pp. 4640–4647.
Lu, C.H., Qi, L.M., Yang, J.H., et al., Adv. Mater., 2005, vol. 17, pp. 2562–2567.
Figueiredo, V., Elangovan, E., Goncalves, G., et al., J. Phys. Status Solidi A, 2009, vol. 206, no. 9, pp. 2143–2148.
Wang, W.Z., Wang, G.H., Wang, X.S., et al., J. Adv. Mater., 2002, vol. 14, no. 1, pp. 67–69.
Li, C.L. and Fu, Z.W., Electrochim. Acta, 2008, vol. 53, no. 12, pp. 4293–4301.
Park, J.C., Kim, J., Kwon, H., and Song, H., Adv. Mater., 2009, vol. 21, pp. 803–807.
Zhang, J., Liu, J., Peng, Q., et al., Chem. Mater., 2006, vol. 18, no. 4, pp. 867–871.
Zhang, H., Zhu, Q., Zhang, Y., et al., Adv. Funct. Mater., 2007, vol. 17, pp. 2766–2771.
Ma, L.L., Li, H.Z., Qiu, M.Q., et al., Mater. Res. Bull., 2010, vol. 45, no. 8, pp. 961–968.
Fernando, C.A.N., Bandara, T.M.W.J., and Wethasingha, S.K., Sol. Energ. Mater. Sol. C, 2001, vol. 70, no. 2, pp. 121–129.
Senevirathna, M.K.I., Pitigala, P.K.D.D.P., Tennakone, K., J. Photochem. Photobiol. A: Chem., 2005, vol. 171, no. 3, pp. 257–259.
Chang, Y., Teo, J.J., and Zeng, H.C., Langmuir, 2005, vol. 21, no. 3, pp. 1074–1079.
Chao, Q., Li, Z., Hui, W., and Jiang, C., J. Phys. Chem. Lett., 2012, vol. 3, no. 5, pp. 651–657.
Liu, H., Ni, Y., Wang, F., et al., Colloids Surf. A, 2004, vol. 235, no. 1–3, pp. 79–82.
Wang, W.Z., Wang, G.H., Wang, X.S., et al., Adv. Mater., 2002, vol. 14, no. 1, pp. 67–69.
Wang, Z., Chen, X., Liu, J., et al., Room, Solid State Commun., 2004, vol. 130, no. 9, pp. 585–589.
Xu, H. and Wang, W., Angew. Chem. Int. Ed., 2007, vol. 46, no. 9, pp. 1489–1492.
Ma, L.L., Li, J.L., Sun, H.Z., et al., Mater. Res. Bull., 2010, vol. 45, no. 8, pp. 961–968.
Wang, Z., Wang, H., Wang, L., and Pan, L., J. Phys. Chem. Solids, 2009, vol. 70, no. 3–4, pp. 719–722.
Deo, M., Shinde, D., Yengantiwar, A., et al., J. Mater. Chem., 2012, vol. 22, no. 33, pp. 17055–17062.
Reda, S.M., Khairy, M., and Mousa, M.A., Arabian J. Chem., 2017, vol. 1, pp. 1–36.
Tao, Sh., Yanga, M., Chena, H., et al., J. Colloid Interface Sci., 2017, vol. 486, pp. 16–26.
Hu, X., Zhou, X., Wang, R., et al., Appl. Catal. B: Environmental, 2014, vol. 154–155, p. 44.
Yang, J., Li, Zhen, Zhao, C., et al., Mater. Res. Bull., 2014, vol. 60, pp. 530–536.
Yadolah, D., The Concise Encyclopedia of Statistics, Springer, 2008.
Ghayyem, M.A., Keyhani, M., Behjoomanesh, M., and Tavakoli, R., Conference and Exhibition, Kuala Lumpur, 05 May 2012.
Sohrabi, S., Akhlaghian, F., Process Safety and Environmental Protection, 2016, vol. 99, pp. 120.
Thomas, H., Salzberg, W., and Thomas, W.J., Academic Press, London, 1967.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Sepahvand, M., Fazaeli, R., Jameh-Bozorghi, S. et al. Enhancing Photocatalytic Activity of Cu2O in Degradation of Sulphonic Acid-Based Dye. Russ J Appl Chem 92, 141–149 (2019). https://doi.org/10.1134/S1070427219010208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427219010208