Abstract
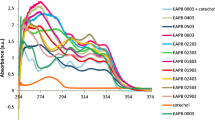
In this study, a series of pyrazoloisoquinoline compounds with novel structures were synthesized by 1,3-dipolar cycloaddition method. The products demonstrate good fluorescence emission and the fluorescent probe molecules potential.




Similar content being viewed by others
REFERENCES
Jin, T.-Y., Li, S.-Q., Jin, C.-R., Shan, H., Wang, R.-M., Zhou, M.-X., Li, A.-L., Li, L.-Y., Hu, S.-Y., and Shen, T., J. Natural Prod., 2018, vol. 81, p. 768. https://doi.org/10.1021/acs.jnatprod.7b00762
Chavez-Santos, R.M., Reyes-Gutierrez, P.E., Torres-Ochoa, R.O., Ramírez-Apan, M., and Martínez, R., Chem. Pharm. Bull., 2017, vol. 65, p. 973. https://doi.org/10.1248/cpb.c17-00409
Jin, T.-Y., Li, S.-Q., Jin, C.-R., Shan, H., Wang, R.-M., Zhou, M.-X., Li, A.-L., Li, L.-Y., Hu, S.-Y., and Shen, T., J. Nat. Prod., 2018, vol. 81, p. 768. https://doi.org/10.1021/acs.jnatprod.7b00762
Singha, R., Dhara, S., and Ray, J.K., Tetrahedron Lett., 2013, vol. 54, p. 4841. https://doi.org/10.1016/j.tetlet.2013.06.092
Lv, Y., Meng, J., Li, C., Wang, X., Ye, Y., and Sun, K., Adv. Synth. Catal., 2021, vol. 363, p. 5235. https://doi.org/10.1002/adsc.202101184
Jayaraman, M., Fox, B.M., Hollingshea, M., Kohlhagen, G., Pommier, Y., and Cushman, M., J. Med. Chem., 2002, vol. 45, p. 242. https://doi.org/10.1021/jm000498f
Zhao, Y.-H., Yu, Y., Hu, D., Zhao, L., Xie, W., and Zhou, Z., Asian J. Org. Chem., 2020, vol. 9, p. 953. https://doi.org/10.1002/ajoc.202000202
Zhao, Y.-H., Luo, Y., Zhu, Y., Wang, H., Zhou, H., Tan, H., Zhou, Z., Ma, Y.-C., Xie, W., and Tang, Z., Synlett, 2018, vol. 29, p. 773. https://doi.org/10.1055/s-0036-1591743
Al-Salahi, R., Abuelizz, H. A., EI Dib, R., and Marzouk, M., Med. Chem., 2017, vol. 13, p. 85. https://doi.org/10.2174/1573406412666160610095706
Mortier, J., Frederick, R., Ganef, C., Remouchamps, C., and Talaga, P., Biochem. Pharmacol., 2010, vol. 79, p. 1462. https://doi.org/10.1016/j.bcp.2010.01.007
Li, Y., Zhao, Y., Luo, M., Tang, Z., Cao, C., and Deng, K., Chin. J. Org. Chem., 2016, vol. 36, p. 2504. https://doi.org/10.6023/cjoc201604031
Newman, D.J. and Cragg, G.M., J. Nat. Prod. 2016, vol. 79, p. 629. https://doi.org/10.1021/acs.jnatprod.5b01055
Freestone, C. and Eccles, R.J.P., J. Pharm. Pharmacol., 1997, vol. 49, p. 1045. https://doi.org/10.1111/j.2042-7158.1997.tb06039.x
Xu, M., Liu, L., Qi, C., Deng, B., and Cai, X., Planta. Med., 2008, vol. 74, p. 1423. https://doi.org/10.1055/s-2008-1081346
Heinrich, M. and Teoh, H.L., J. Ethnopharmacol., 2004, vol. 92, p. 147. https://doi.org/10.1016/j.jep.2004.02.012
Luo, J.L., Jin, T., Váradi, L., Perry, J.D., Hibbs, D.E., Groundwater, P.W. Dyes Pigm., 2016, vol. 125, p. 15. https://doi.org/10.1016/j.dyepig.2015.09.031
Singh, S., Pathak, N., Fatima, E., and Negi, A.S., Eur. J. Med. Chem., 2021, vol. 226, p. 113839. https://doi.org/10.1016/j.ejmech.2021.113839
Sloop, J.C., J. Chem., 2017, vol. 2017, Article 2860123. https://doi.org/10.1155/2017/2860123
Tang, Y., Yu, Y., Wei, X., Yang, J., Zhu, Y., Zhao, Y.H., Tang, Z., Zhou, Z., Li, X., and Yu, X., Tetrahedron Lett., 2019, vol. 60, p. 151187. https://doi.org/10.1016/j.tetlet.2019.151187
Zhao, Y. H., Yu, Y., Guo, T., Zhou, Z., Tang, Z., and Tian, L., Dyes Pigm., 2020, vol. 181, p. 108547. https://doi.org/10.1016/j.dyepig.2020.108547
Zhu, Y., Yu, Y., Zhao, Y.H., Tang, Z., and Tian, L., Russ. J. Gen. Chem., 2020, vol. 90, p. 1518. https://doi.org/10.1134/S1070363220080204
Xu, J., Zhang, Y., Yu, H., Gao, X., and Shao, S., Anal. Chem., 2016, vol. 88, p. 1455. https://doi.org/10.1021/acs.analchem.5b04424
Zhao, Y.-H., Luo, Y., Wang, H., Wei, H., Guo, T., Tan, H., Yuan, L., and Zhang, X.-B., Anal. Chim. Acta, 2019, vol. 1065, p. 134. https://doi.org/10.1016/j.aca.2019.03.029
Yu, Y., Guan, M., Zhao, Y.-H., Xie, W., Zhou, Z., and Tang, Z., Russ. J. Gen. Chem., 2020, vol. 90, p. 2012. https://doi.org/10.1134/S1070363220100266
Paronikyan, E.G., Dashyan, S., and Stepanyan, H.M., Russ. J. Org. Chem., 2020, vol. 56, p. 1963. https://doi.org/10.1134/S1070428020110111
Zhao, Y.-H., Li, Y., Luo, M., Tang, Z., and Deng, K., Synlett, 2016, vol. 27, p. 2597. https://doi.org/10.1055/s-0035-1562609
Zhao, Y.-H., Luo, M., Li, Y., Liu, X., Tang, Z., Deng, K., and Zhao, G., Chin. J. Chem., 2016, vol. 34, p. 857. https://doi.org/10.1002/cjoc.201600277
Zhao, Y.-H., Li, Y., Guo, T., Tang, Z., Xie, W., and Zhao, G., Tetrahedron Lett., 2016, vol. 57, p. 2257. https://doi.org/10.1016/j.tetlet.2016.04.037
Yang, J., Wei, X., Yu, Y., Zhu, Y., Zhao, Y.-H., Xie, W., and Zhao, L., Tetrahedron Lett., 2020, vol. 61, p. 151454. https://doi.org/10.1016/j.tetlet.2019.151454
Zhao, Y.-H., Li, Y., Guo, T., Tang, Z., Deng, K., and Zhao, G., Synth. Commun., 2016, vol. 46, p. 355. https://doi.org/10.1080/00397911.2015.1137944
Funding
The generous financial support from the National Natural Science Foundation of China (no. 21877034), Natural Science Foundation of Hunan Provincial (no. 2020JJ4028), Postgraduate Scientific Research Innovation Project of Hunan Province (CX20201000) and the Science and Technology Foundation of Henan Province (no. 212102210202). are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No confl ict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Yu, Y., Zheng, P., Tan, J. et al. Synthesis and Fluorescence Properties of Novel Pyrazolo-Isoquinoline Compounds. Russ J Gen Chem 92, 1360–1366 (2022). https://doi.org/10.1134/S107036322207026X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322207026X