Abstract
A method for the synthesis of 5-phenyl-1,3-thiazole-4-sulfonyl chloride was developed based on the cyclization of ethyl 2-{[1-(benzylsulfanyl)-2-oxo-2-phenylethyl]amino}-2-oxoacetate obtained from available reagents under the action of the Lawesson’s reagent and oxidative chlorination of the intermediate benzyl 5-phenyl-1,3thiazol-4-ylsulfide. The resulting sulfonyl chloride was converted into a series of 5-phenyl-1,3-thiazole-4-sulfonamide derivatives for which in vitro antitumor activity screening studies were performed on 60 cancer cell lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Malignant diseases are widespread. They are considered as the main problem of this century, which worries the medical community around the world [1]. The development of resistance to anticancer drugs, leading to their inefficiency, necessitates the search for new highly active and less toxic chemotherapeutic agents. Among drug development strategies, special attention is paid to molecules containing heterocycles with a sulfur atom in their structure, and, in particular, a thiazole ring, since its derivatives have excellent pharmacological characteristics [2–4]. The thiazole ring is present in more than 18 FDA-approved drugs [4].
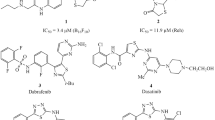
Modification of a thiazole scaffold with carboxyl, sulfonyl, or sulfonamide pharmacophores significantly expands the spectrum of biological activity, making such hybrids ideal candidates for the development of more effective and safer drugs. Among them, the most studied are thiazole-4- and thiazole-5-carboxylic acid derivatives [2, 5, 6], which include the well-known antitumor drugs bleomycin 1 and dasatinib 2 (Scheme 1). Among thiazole-2-carboxylic acid amides 3, effective inhibitors of ubiquitin-specific peptidase 7 (USP7) were found [7].
Sulfonyl hybrids are widely studied for their anticancer activity, as they are characterized by minimal side effects along with encouraging indicators of the possible development of drug resistance associated with their repeated use [8–10]. Thiazolesulfonamides have a wide spectrum of pharmacological activity, among which a number of thiazole-4- (4) and thiazole-5-sulfonamides (5) have been proposed for the prevention or treatment of cancer as inhibitors of ATM and DNA-PK [11], PI3Kα [12], Raf [13] kinases and ATG4B protease [14].
This work is a continuation of our research on the synthesis of thiazole-4-sulfonamides [15] in order to search for effective anticancer agents, and is based on the idea of creating previously unknown sulfonyl hybrids of thiazolecarboxylic acids.
To implement this task, we used an approach developed by us earlier [15–17] based on the cyclization of N-(2-oxo-2-arylethyl)amides under the action of 2,4-bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetan-2,4-dithione (Lawesson’s reagent) and oxidative chlorination of benzylsulfanyl-1,3-thiazoles. Ethyl 2-[(1-hydroxy-2-oxo-2-phenylethyl)amino]-2-oxoacetate 6 obtained by heating a mixture of reagents in tetrahydrofuran (Scheme 2). Further, using a simple sequence 6→7→8, carbinol 6 was converted into ethyl 2-{[1-(benzylsulfanyl)-2-oxo-2-phenylethyl]amino}-2-oxoacetate 8. 1-Substituted 2-[(2-phenyl-2-oxoethyl)amino]-2-oxoacetates 6–8 were obtained for the first time. Their spectral characteristics fully correspond to the expected ones and agree with the data for the previously described N-(2-aryl-1-R-2-oxoethyl)carboxamides with similar structures [15, 17].
Thionization of oxoacetate 8 with Lawesson’s reagent was carried out in anhydrous dioxane, resulting in cyclization to the 1,3-thiazole derivative. To isolate product 9, we applied a procedure previously used in the synthesis of 4-substituted 1,3-thiazoles: treatment of the reaction mixture with an aqueous NaOH solution followed by extraction of the product with chloroform [15–17]. However, according to IR spectroscopy data (the absence of strong stretching vibrations of the CO2Et group), the decarboxylation product—benzyl-5-phenyl1,3-thiazol-4-ylsulfide 10 – was isolated instead of the expected ethyl 4-(benzylsulfanyl)-5-phenyl-1,3-thiazole-2-carboxylate 9. It should be noted that the synthesis of 4-unsubstituted ethyl 5-aryl-1,3-thiazol-2-carboxylates has been successfully carried out earlier under similar conditions [18].
Since compound 10 could not be isolated in pure form, we tried to obtain it in another way, starting from the adduct of formamide and phenylglyoxal. However, unlike 4-chlorophenyl- and 4-methylphenylglyoxals, for which adducts with formamide have been successfully synthesized [17, 19], the reaction of phenylglyoxal with formamide was accompanied by significant resin formation, regardless of conditions (when heated or at room temperature, in benzene or THF).
The crude product 10 was used to obtain 5-phenyl-1,3-thiazole-4-sulfonyl chloride 11. Compound 11 was synthesized by oxidative chlorination in acetic acid.
Amination of sulfonyl chloride 11 with ammonia and amines was carried out by heating in dioxane (Scheme 3), which made it possible to obtain a series of sulfonamides 12–15 for further testing for anticancer activity. It should be noted that the list of 2-unsubstituted 1,3-thiazole-4-sulfonyl chlorides and 4-sulfonamides described earlier is limited to 5-unsubstituted derivatives and compounds with non-aromatic substituents in position 5 [11, 20, 21]. For reactions, amines containing pharmacophore fragments (piperidine, morpholine, piperazine or tetrahydrothiophene-1,1-dione) were chosen, since such structural fragments are present in a number of azole sulfonamides, which exhibit a high level of anticancer activity [22, 23].
Amination product 12 was formed by the reaction of sulfonyl chloride 11 with aqueous ammonia at room temperature. N-Substituted sulfonamides 13–15 were obtained by heating a reactants mixture with triethylamine in dioxane. The yields of amination products are practically independent of the amine structure; all sulfonamides were obtained in high yields (70–85%).
Composition and structure of new thiazole derivatives 11–15 were confirmed by elemental analysis, IR, 1H and 13C NMR spectroscopy, and mass spectrometry methods. The formation of an azole ring is indicated by the absence in the IR and NMR spectra of the NH signals and carbonyl groups characteristic of the starting acyclic compound 9. Stretching vibrations of the SO2 group in the IR spectra of compounds 11–15 are observed in the form of two strong bands in the 1143–1193 and 1332–1382 cm–1 regions. A characteristic feature of the 1H NMR spectra of thiazoles 11–15 is the presence of a singlet of the aromatic proton of the C2H thiazole ring in a downfield (9.11–9.29 ppm). In the 13C NMR spectra, the resonance of the tertiary carbon С2 of the thiazole ring is observed in a lower field (153.5–155.1 ppm) than the signal of the quaternary carbon С4 (143.6–150.1 ppm).
As part of the international scientific program of the US National Institutes of Health, testing of the antitumor activity of thiazole-4-sulfonamides 12–15 was carried out. Screening studies were carried out in vitro on 60 cancer cell lines that cover current types of human cancers (lines of lung, kidney, CNS, ovarian, prostate, breast, epithelial cancer, as well as leukemia and melanoma) when exposed to a substance at a concentration of 1× 10–5 M, as a result of which the growth percentage (GP) of cancer cell lines was determined in comparison with the control (control, 100%) [24–27].
It was fond that the level of antitumor activity of 5-phenyl-1,3-thiazole-4-sulfonamides 12–15 determines the structure of the amine fragment. Compounds 13, 14d, and 15a–15d, which contain bulky sulfonamide groups in their structure, showed the lowest activity, inhibiting the growth of cancer cells by less than 20% (GP > 80%). The activity of piperazine derivatives 14g–14k is somewhat higher, except for 1-(4-methoxyphenyl)piperazine 14i (Table 1). At the same time, only some cell lines were moderately sensitive to the action of compounds 14g, 14h and 14j, 14k (GP < 80%). The best indicator in this group of thiazolyl-4-sulfonamides was found for the 1-(3-chlorophenyl)piperazine derivative 14h, which reduced the growth of colon cancer cell line HT29 to 68.13%.
Amino-unsubstituted thiazolyl-4-sulfonamide 12 and 2,6-dimethylmorpholinyl derivative 14f had a moderate inhibitory effect (GP < 80%) on the growth of SNB-75 (CNS) and UO-31 (kidney cancer, 14f) cell lines.
Among the tested thiazolyl-4-sulfonamides, 4-methyl-1-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]piperidine 14c showed the highest activity (Table 1). Thus, a noticeable decrease in the growth of most leukemia cancer cells (GP 25.52–66.75%) and CNS (GP 30.71–57.90%) was found. In relation to subpanels of kidney cancer and breast cancer, compound 14c was more selective. At the same time, the rate of inhibition of the renal line RFX 393 exceeded 90% (GP 9.28%). Also, the renal line 786-0 (GP 30.81%) and HS 578T (GP 40.25%) of breast cancer have a high sensitivity to thiazole 14c.
In conclusion, a method was proposed for the synthesis of 5-phenyl-1,3-thiazole-4-sulfonyl chloride and 5-phenyl-1,3-thiazole-4-sulfonamides, new promising antitumor agents, the high level of activity of which was experimentally confirmed on 60 tumor cell lines. The method is based on a synthetic sequence involving cyclization of ethyl 2-{[1-(benzylsulfanyl)-2-oxo-2-phenylethyl]amino}-2-oxoacetate obtained from available reagents under the action of Lawesson's reagent.
EXPERIMENTAL
All reagents and solvents used in synthetic procedures were purchased from Aldrich and used without further purification.
1H and 13C NMR spectra were recorded on a Varian Mercury 400 spectrometer (400 and 126 MHz). The internal standard was TMS. The signals of 13C atoms (methyl, methylene, methine, and quaternary carbon atoms) were assigned using the APT method, taking into account the known range of chemical shifts of carbon atoms included in the functional groups. IR spectra (KBr) were recorded on a Bruker VERTEX 70 instrument. Mass spectra were recorded on an Agilent 1200 LCMCD SL instrument [chemical ionization (APCI), electrospray ionization (ESI)]. Melting points were determined on a Fisher-Johns apparatus. Elemental analyzes were performed at the Analytical Laboratory of the Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine. The content of carbon and hydrogen was determined by the Pregl gravimetric method, nitrogen—by the gravimetric Dumas micromethod, sulfur—by the Schöniger titrimetric method, and chlorine—by the mercurometric method.
Ethyl 2-[(1-hydroxy-2-oxo-2-phenylethyl)amino]-2-oxoacetate (6). To a solution of 67.0 g (0.5 mol) of freshly distilled phenylglyoxal in 250 mL of THF was added 58.5 g (0.5 mol) of ethyl 2-amino-2-oxoacetate. The resulting solution was boiled for 6 h and then kept at 20–25°С for 12 h. The solvent was removed in vacuum and 500 mL of water was added to the residue. The resulting solid was filtered off, washed with water and dried in a desiccator over P2O5. Compound 6 was purified by recrystallization from ethanol. Yield 94.26 g (75%), beige crystals, mp 99–101°С (EtOH). IR spectrum, ν, cm–1: 703, 751, 1283 m (C–O), 1400 s (C–O), 1579 s (C=O), 1687 m (C=O), 3364 br (NH), 3422 br (ОН). 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.26 t (3Н, ОСН2СН3, 3JНН 7.5 Hz), 4.24 q (2Н, ОСН2СН3, 3JНН 7.5 Hz), 6.30 d (1Н, С1Н, 3JНН 6.0 Hz), 6.83 br. s (1Н, ОН), 7.54 t (2Н, С3′H, C5′H, 3JНН 8.0 Hz), 7.66 t (1Н, С4′H, 3JНН 8.0 Hz), 7.97 d (2Н, С2′H, C6′H, 3JНН 8.0 Hz), 9.45 d (1Н, NН, 3JНН 6.0 Hz). 13С NMR spectrum (DMSO-d6), δC, ppm: 13.8 (СН3), 62.3 (ОСН2), 73.0 (С(Н)ОН), 128.7 (2С), 128.8 (2С), 133.7, 133.8, 157.1 (СОСО2Et), 160.4 (СОСО2Et), 194.0 (COPh). Mass spectrum, m/z (Irel, %): 252.1 (100) [M + H]+. Found, %: С 57.43; Н 5.20; N 5.54. C12H13NO5. Calculated, %: С 57.37; Н 5.22; N 5.58.
Ethyl 2-[(1-chloro-2-oxo-2-phenylethyl)amino]2-oxoacetate (7). To a solution of 100.4 g (0.4 mol) of compound 6 in 250 mL of anhydrous THF was added 32.6 mL (0.45 mol) of thionyl chloride. The solution was stirred at 20–25°C for 24 h. The solvent was removed in vacuum and 200 mL of anhydrous hexane was added to the residue. The solid was filtered off, washed with hexane and dried in vacuum. Yield 86.3 g (80%), colorless crystals, mp 48–50°С (hexane). IR spectrum, ν, cm–1: 611, 688, 805, 1181 s (C–O), 1289 br. s (C–O), 1506, 1687 s (C=O), 1710 s (C=O), 1743 s (C=O), 2995, 3343 (NH). 1Н NMR spectrum (CDCl3), δ, ppm: 1.43 t (3Н,ОСН2СН3, 3JНН 7.5 Hz), 4.43 q (2Н, ОСН2СН3, 3JНН 7.5 Hz), 7.07 d ( 6.47 d (1Н, СHCl, 3JНН 9.0 Hz), 7.56 t (2Н, С3′H, C5′H, 3JНН 8.0 Hz), 7.70 t (1Н, С4′H, 3JНН 8.0 Hz), 8.10 d (2Н, С2′H, C6′H, 3JНН 8.0 Hz), 8.68 br. d (1Н, NН, 3JНН 9.0 Hz). 13С NMR spectrum (CDCl3), δC, ppm: 13.5 (СН3), 59.1 (ОСН2), 63.4 (СHCl), 128.7 (2С), 129.1 (2С), 131.1, 134.6, 155.4 (СОСО2Et), 158.5 (СОСО2Et), 186.7 (COPh). Mass spectrum, m/z (Irel, %): 270.5 (100) [M + H]+. Found, %: С 53.50; Н 4.45; Cl 13.26; N 5.16. C12H12ClNO4. Calculated, %: С 53.44; Н 4.49; Cl 13.15; N 5.19.
Ethyl 2-{[1-(benzylsulfanyl)-2-oxo-2-phenylethyl]amino}-2-oxoacetate (8). To a solution of 80.7 g (0.3 mol) of compound 7 in 200 mL of anhydrous acetonitrile was added 37.2 g (0.3 mol) of benzyl mercaptan and 42 mL (0.3 mol) of triethylamine. The solution was heated to boiling and left for 12 h at 20–25°C, then 500 mL of water was added. The formed precipitate was filtered off and washed with water. Compound 8 was purified by recrystallization from ethanol. Yield 83.6 g (78%), pale yellow crystals, mp 109–111°С (EtOH). IR spectrum, ν, cm–1: 704, 766, 977, 1194 m (С–О), 1285 s (С–О), 1679 s (С=О), 1704 s (С=О), 1735 s (С=О), 3348 m (NH). 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.29 t (3Н, ОСН2СН3, 3JНН 7.5 Hz), 3.81 d (1Н, SCHAHB, 2JНН 14.0 Hz), 3.94 d (1Н, SCHAHB, 2JНН 14.0 Hz), 4.26 q (2Н,ОСН2СН3, 3JНН 7.5 Hz), 6.47 d (1Н, С1Н, 3JНН 8.0 Hz), 7.28–7.31 m (5Н, SCH2Ph), 7.47 t (2Н, С3′H, C5′H, 3JНН 8.0 Hz), 7.65 t (1Н, С4′H, 3JНН 8.0 Hz), 7.86 d (2Н, С2′H, C6′H, 3JНН 8.0 Hz), 9.32 d (1Н, NН, 3JНН 8.0 Hz). 13С NMR spectrum (DMSO-d6), δC, ppm: 13.8 (СН3), 33.9 (SCH2Ph), 56.1 (SC(H)NH), 62.3 (ОСН2), 127.2, 128.5 (2C), 128.7 (2C), 128.7 (2C), 129.1 (2C), 133.4, 133.9, 137.1, 157.1 (СОСО2Et), 160.1 (СОСО2Et), 190.9 (COPh). Mass spectrum, m/z (Irel, %): 358.1 (100) [M + H]+. Found, %: С 63.92; Н 5.34; N 3.89; S 9.01. C19H19NO4S. Calculated, %: С 63.85; Н 5.36; N 3.92; S 8.97.
5-Phenyl-1,3-thiazole-4-sulfonyl chloride (11). A mixture of 89.2 g (0.25 mol) of oxoacetate 8 and 101 g (0.25 mol) of Lawesson's reagent in 200 mL of anhydrous dioxane was refluxed for 8 h. Then the mixture was kept at 20–25°С for 12 h. The solvent was removed in vacuum. To the residue was added 100 mL of 5% NaOH aqueous solution. The reaction product was extracted from the resulting oil with chloroform (2×100 mL), the extract was dried with CaCl2. The solvent was removed in vacuum and the residue was dissolved in 100 mL of ethanol. To a solution containing ethyl 4-(benzylsulfanyl)-5-phenyl-1,3-thiazole-2-carboxylate 9 was added 100 mL of 10% NaOH aqueous solution, boiled for 1 h and left at 20–25°C for 2 h. Next, hydrochloric acid (conc.) was added to the solution to pH 2 and boiled for 1 h. After cooling, the solvent was removed in vacuum and 200 mL of water was added to the residue. The product was extracted with chloroform (2×100 mL), the extract was dried with CaCl2. The solvent was removed in vacuum, the residue was dissolved in 80 mL of acetic acid (95%), and gaseous Cl2 was bubbled through the solution containing benzyl-5-phenyl-1,3-thiazol-4-ylsulfide 10 for 30 min, maintaining the temperature of the reaction mixture within 0–5°С. Next, the solution was kept at 0–5°C for 2 h and then poured into ice (500 g). The formed precipitate was filtered off and dried in a vacuum desiccator over Р2О5. Compound 11 was purified by recrystallization from toluene. Yield 42.2 g (65%), colorless crystals, mp 114–116°С (toluene). IR spectrum, ν, cm–1: 555, 571, 756, 1193 s (SO2), 1382 s (SO2), 1424, 3096. 1НNMR spectrum (CDCl3), δ, ppm: 7.51–7.55 m (3Н, H-Ar), 7.58 d (2Н, С2′H, C6′H, 3JНН 8.0 Hz), 8.89 s (1Н, С2Н). 1Н NMR spectrum (DMSO-d6), δ, ppm: 7.37–7.39 m (3Н, 5-Ph), 7.73–7.76 m (2Н, 5-Ph), 9.11 s (1Н, С2Н). 13С NMR spectrum (CDCl3), δC, ppm: 126.3, 128.2 (2C), 29.8 (2C), 130.2, 146.0 (C4), 152.0 (C2). Mass spectrum, m/z (Irel, %): 260.6 (100) [M + H]+. Found, %: С 41.68; Н 2.32; N 5.35; S 24.72. C9H6ClNO2S2. Calculated, %: С 41.62; Н 2.33; N 5.39; S 24.69.
5-Phenyl-1,3-thiazole-4-sulfonamide (12). A solution of 2.59 g (0.01 mol) of sulfonyl chloride 11 in 30 mL of anhydrous dioxane was added dropwise with stirring and cooling (0°С) to a 25% aqueous ammonia solution (70 mL). The resulting suspension was stirred for 30 min at 20–25°C. The solid was filtered off and washed with water. Compound 12 was purified by recrystallization from ethanol. Yield 2.04 g (85%), colorless crystals, mp 166–168°С (EtOH). IR spectrum, ν, cm–1: 515, 603, 655, 696, 759, 1147 s (SO2), 1183, 1275, 1344 s (SO2), 1425, 1556, 3150 m (NH2), 3342 m (NH2). 1Н NMR spectrum (DMSO-d6), δ, ppm: 7.45–7.46 m (3Н, 5-Ph), 7.58–7.60 m (2Н, 5-Ph), 7.65 s (2Н, NH2), 9.20 s (1Н, С2Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 128.2 (2C), 129.0, 129.2, 130.2 (2C), 139.5, 150.1 (C4), 153.5 (C2). Mass spectrum, m/z (Irel, %): 240.0 (100) [M + H]+. Found, %: С 45.03; Н 3.34; N 11.65; S 26.72. C9H8N2O2S2. Calculated, %: С 44.98; Н 3.36; N 11.66; S 26.69.
General procedure for the synthesis of 5-phenyl-1,3-thiazole-4-sulfonamide derivatives 13–15. To a solution of 2.59 g (0.01 mol) of sulfonyl chloride 11 in 30 mL of anhydrous dioxane was added 0.01 mol of the corresponding aniline or dialkylamine and 1.4 mL (0.01 mol) of triethylamine. The mixture was boiled for 2 h and left at 20–25°С for 12 h. The solvent was removed in a vacuum, and 50 mL of water was added to the residue. The solid was filtered off, washed with water and purified by crystallization from ethanol.
N-[4-(4-Morpholinylsulfonyl)phenyl]-5phenyl-1,3-thiazole-4-sulfonamide (13). Yield 3.35 g (72%), colorless crystals, mp 181–183°С (EtOH). IR spectrum, ν, cm–1: 533, 585, 603, 741, 831, 935, 1109 m (C–O–C), 1142 s (SO2), 1165 s (SO2), 1345 s (SO2), 1596, 3236 s (NH). 1Н NMR spectrum (DMSO-d6), δ, ppm: 2.80 t (4Н, NСН2, 3JНН 4.0 Hz), 3.61 t (4Н, OСН2, 3JНН 4.0 Hz), 7.24 d (2Н, С2′H, C6′H, 3JНН 8.0 Hz), 7.50 br. s (5Н, 5-Ph), 7.60 d (2Н, С3′H, C5′H, 3JНН 8.0 Hz), 9.19 s (1Н, С2Н), 11.29 s (1Н, NH). 13С NMR spectrum (DMSO-d6), δC, ppm: 45.9 (2C, NCH2), 65.2 (2C, OCH2), 118.5 (2C), 128.4 (3C), 128.5, 129.1 (2C), 129.7, 130.1 (3C), 142.5, 143.6, 154.8 (C2). Mass spectrum, m/z (Irel, %): 466.3 (100) [M + H]+. Found, %: С 49.07; Н 4.09; N 9.05; S 20.71. C19H19N3O5S3. Calculated, %: С 49.02; Н 4.11; N 9.03; S 20.66.
1-[(5-Phenyl-1,3-thiazol-4-yl)sulfonyl]piperidine (14a). Yield 2.41 g (78%), colorless crystals, mp 117–119°С (EtOH). IR spectrum, ν, cm–1: 586, 719, 826, 938, 1051, 1143 s (SO2), 1158, 1189, 1348, 1361 s (SO2), 2930, 3049. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.45–1.52 m (6Н, С3Н2–С5Н2), 3.17–3.19 m (4Н, С2Н2, С6Н2), 7.46–7.48 m (3Н, 5′-Ph), 7.53–7.54 m (2Н, 5′-Ph), 9.25 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 23.1 (C4H2), 25.0 (2C, C3H2, C5H2), 46.8 (2C, NCH2), 128.2, 128.6 (2C), 129.4, 130.2 (2C), 142.7, 146.3 (C4′), 154.5 (C2′). Mass spectrum, m/z (Irel, %): 309.2 (100) [M + H]+. Found, %: С 54.58; Н 5.20; N 9.11; S 20.75. C14H16N2O2S2. Calculated, %: С 54.52; Н 5.23; N 9.08; S 20.79.
3-Methyl-1-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]piperidine (14b). Yield 2.74 g (85%), colorless crystals, mp 105–107°С (EtOH). IR spectrum, ν, cm–1: 594, 745, 1145 s (SO2), 1350 s (SO2), 1425, 2934. 1Н NMR spectrum (DMSO-d6), δ, ppm: 0.84 d (3Н, СН3, 3JНН 8.0 Hz), 0.93–0.99 m (1Н, С4НАНВ), 1.38–1.47 m (1Н, С4НАНВ), 1.54–1.58 m (1Н, С3Н), 1.65–1.69 m (2Н, С5Н2), 2.41–2.50 m (1Н, С6НАНВ), 2.67–2.76 m (1Н, С6НАНВ), 3.50–3.56 m (2Н, С2Н2), 7.47–7.48 m (3Н, 5′-Ph), 7.53–7.55 m (2Н, 5′-Ph), 9.25 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 21.0 (CH3), 24.4 (C5H2), 30.4 (C4H2), 31.6 (C3H), 46.4 (NC6H2), 53.0 (NC2H2), 128.2 (2C), 128.6, 129.4, 130.2 (2C), 141.4, 142.7 (C4′), 154.5 (C2′). Mass spectrum, m/z (Irel, %): 323.1 (100) [M + H]+. Found, %: С 55.93; Н 5.59; N 8.67; S 19.92. C15H18N2O2S2. Calculated, %: С 55.87; Н 5.63; N 8.69; S 19.89.
4-Methyl-1-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]piperidine (14c). Yield 2.58 g (80%), colorless crystals, mp 92–94°С (EtOH). IR spectrum, ν, cm–1: 583, 726, 919, 1144 s (SO2), 1185, 1347 m (SO2), 1359, 1420, 2925. 1Н NMR spectrum (DMSO-d6), δ, ppm: 0.88 d (3Н, СН3, 3JНН 8.0 Hz), 1.02–1.13 m (2Н, С3НАНВ, С5НАНВ), 1.38–1.47 m (1Н, С4Н), 1.62–1.67 m (2Н, С3НАНВ, С5НАНВ), 2.73–2.80 m (2Н, С2НАНВ, С6НАНВ), 3.59–3.64 m (2Н, С2НАНВ, С6НАНВ), 7.46–7.48 m (3Н, 5′-Ph), 7.53–7.55 m (2Н, 5′-Ph), 9.25 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 21.4 (CH3), 29.6 (C4H), 33.1 (2C, C3H2, C5H2), 46.2 (2C, NCH2), 128.2 (2C), 128.6, 129.4, 130.2 (2C), 142.7, 146.2 (C4′), 154.5 (C2′). Mass spectrum, m/z (Irel, %): 323.0 (100) [M + H]+. Found, %: С 55.92; Н 5.60; N 8.66; S 19.93. C15H18N2O2S2. Calculated, %: С 55.87; Н 5.63; N 8.69; S 19.89.
2-[(5-Phenyl-1,3-thiazol-4-yl)sulfonyl]-1,2,3,4-tetrahydroisoquinoline (14d). Yield 2.50 g (70%), colorless crystals, mp 129–131°С (EtOH). IR spectrum, ν, cm–1: 591, 701, 728, 758, 827, 960, 1024, 1073, 1147 s (SO2), 1332 s (SO2), 1413, 1449, 2836, 3076. 1Н NMR spectrum (DMSO-d6), δ, ppm: 2.85 t (2Н, С4Н2, 3JНН 6.0 Hz), 3.54 t (2Н, С3Н2, 3JНН 6.0 Hz), 4.45 s (2Н, С1Н2), 7.14–7.17 m (4Н, С5Н–С8Н), 7.48–7.50 m (3Н, 5′-Ph), 7.56–7.59 m (2H, 5′-Ph), 9.21 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 28.4 (C4H2), 44.0 (NC3H2), 47.3 (NC1H2), 126.2, 128.3, 128.5 (2C), 128.7, 129.6, 130.2 (2C), 131.8, 133.3, 143.0, 146.2 (C4′), 154,7 (C2′). Mass spectrum, m/z (Irel, %): 357.0 (100) [M + H]+. Found, %: С 60.71; Н 4.50; N 7.81; S 18.04. C18H16N2O2S2. Calculated, %: С 60.65; Н 4.52; N 7.86; S 17.99.
4-[(5-Phenyl-1,3-thiazol-4-yl)sulfonyl]morpholine (14e). Yield 2.36 g (76%), colorless crystals, mp 125–127°С (EtOH). IR spectrum, ν, cm–1: 585, 726, 951, 1110 s (C–O–C), 1151 s (SO2), 1258, 1353 s (SO2), 1421, 1445, 2972, 3063. 1Н NMR spectrum (DMSO-d6), δ, ppm: 3.18–3.21 m (4Н, С3Н2, С5Н2), 3.60–3.63 m (4Н, С2Н2, С6Н2), 7.45–7.56 m (5Н, 5′-Ph), 9.28 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 46.2 (2C, NCH2), 65.7 (2C, OCH2), 128.3 (2C), 128.4, 129.5, 130.2 (2C), 143.5, 145.5 (C4′), 154.8 (C2′). Mass spectrum, m/z (Irel, %): 311.0 (100) [M + H]+. Found, %: С 50.37; Н 4.51; N 9.00; S 20.71. C13H14N2O3S2. Calculated, %: С 50.30; Н 4.55; N 9.03; S 20.66.
2,6-Dimethyl-4-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]morpholine (14f). Yield 2.84 g (84%), colorless crystals, mp 125–127°С (EtOH). IR spectrum, ν, cm–1: 594, 1013, 1082 m (C–O–C), 1154 s (SO2), 1351 s (SO2), 1421, 2870, 2971, 3081. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.07 d (6Н, СН3, 3JНН 8.0 Hz), 2.49–2.54 m (2Н, С3НAHB, С5НAHB), 3.48–3.51 m (2Н, С3НAHB, С5НAHB), 3.55–3.57 m (2Н, C2H, C6H), 7.47–7.49 m (3Н, 5′-Ph), 7.55–7.57 m (2Н, 5′-Ph), 9.28 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 18.4 (CH3), 50.8 (2C, NCH2), 70.9 (2C, OCH), 128.3 (2C), 128.4, 129.6, 130.2 (2C), 143.3, 145.8 (C4′), 154.8 (C2′). Mass spectrum, m/z (Irel, %): 157.0 (40), 339.0 (100) [M + H]+. Found, %: С 53.29; Н 5.33; N 8.25; S 18.99. C15H18N2O3S2. Calculated, %: С 53.23; Н 5.36; N 8.28; S 18.95.
1-Phenyl-4-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]piperazine (14g). Yield 3.12 g (81%), colorless crystals, mp 145–147°С (EtOH). IR spectrum, ν, cm–1: 549, 585, 755, 947, 1150 s (SO2), 1241, 1349 s (SO2), 1504, 1598, 2823, 3082. 1Н NMR spectrum (DMSO-d6), δ, ppm: 3.17–3.20 m (2Н, С3Н, С5Н), 3.34–3.36 m (4Н, С2Н2, С6Н2), 6.82 t (1Н, С4′′H, 3JНН 6.0 Hz), 6.94 d (2Н, С2′′H, С6′′H, 3JНН 8.0 Hz), 7.22 t (2Н, С3′′H, С5′′H, 3JНН 8.0 Hz), 7.48–7.49 m (3Н, 5′-Ph), 7.56–7.59 m (2Н, 5′-Ph), 9.28 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 46.0 (2C, SO2NCH2), 48.3 (2C, PhNCH2), 116.2 (2C), 119.6, 128.3 (2C), 128.4, 129.0 (2C), 129.5, 130.2 (2C), 143.4, 145.7 (C4′), 150.5, 154.8 (C2′). Mass spectrum, m/z (Irel, %): 386.2 (100) [M + H]+. Found, %: С 59.28; Н 4.93; N 10.85; S 16.69. C19H19N3O2S2. Calculated, %: С 59.20; Н 4.97; N 10.90; S 16.64.
1-(3-Chlorophenyl)-4-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]piperazine (14h). Yield 3.28 g (78%), colorless crystals, mp 154–156°С (EtOH). IR spectrum, ν, cm–1: 586, 731, 952, 1150 s (SO2), 1240, 1355 s (SO2), 1490, 1593, 2839, 3087. 1Н NMR spectrum (DMSO-d6), δ, ppm: 3.23–3.26 m (4Н, С3Н2, С3Н2), 3.32–3.34 m (4Н, С2Н2, С6Н2), 6.82 d (1Н, С6′′H, 3JНН 8.0 Hz), 6.90 d (1Н, С4′′H, 3JНН 8.0 Hz), 6.96 s (1Н, С2′′H), 7.22 t (1Н, С5′′H, 3JНН 8.0 Hz), 7.48–7.49 m (3Н, 5′-Ph), 7.56–7.58 m (2Н, 5′-Ph), 9.27 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 45.9 (2C, SO2NCH2), 47.7 (2C, ArNCH2), 114.3, 115.3, 118.8, 128.3 (2C), 128.4, 129.6, 130.2 (2C), 130.5, 133.8, 143.4, 145.6 (C4′), 151.7, 154.8 (C2′). Mass spectrum, m/z (Irel, %): 421.0 (100) [M + H]+. Found, %: С 54.41; Н 4.35; N 9.94; S 15.32. C19H18ClN3O2S2. Calculated, %: С 54.34; Н 4.32; N 10.01; S 15.27.
1-(4-Methoxyphenyl)-4-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]piperazine (14i). Yield 3.16 g (76%), colorless crystals, mp 151–153°С (EtOH). IR spectrum, ν, cm–1: 585, 733, 823, 948, 1150 s (SO2), 1248 s (C–O), 1355 s (SO2), 1514, 2833, 3082. 1Н NMR spectrum (DMSO-d6), δ, ppm: 3.05–3.06 m (4Н, С3Н2, С5Н2), 3.33–3.34 m (4Н, С2Н2, С6Н2), 3.68 s (3Н, ОСН3), 6.82 d (2Н, С3′′H, С5′′H, 3JНН 8.0 Hz), 6.90 d (2Н, С2′′H, С6′′H, 3JНН 8.0 Hz), 7.47–7.49 m (3Н, 5′-Ph), 7.56–7.58 m (2Н, 5′-Ph), 9.28 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 46.2 (2C, SO2NCH2), 49.7 (2C, ArNCH2), 55.2 (OCH3), 114.3 (2C), 118.3 (2C), 128.3 (2C), 128.4, 129.5, 130.2 (2C), 143.0, 1144.9, 145.6 (C4′), 153.6, 154.8 (C2′). Mass spectrum, m/z (Irel, %): 416.2 (100) [M + H]+. Found, %: С 57.90; Н 5.04; N 10.08; S 15.48. C20H21N3O3S2. Calculated, %: С 57.81; Н 5.09; N 10.11; S 15.43.
1-(4-Fluorophenyl)-4-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]piperazine (14j). Yield 3.35 g (83%), colorless crystals, mp 121–123°С (EtOH). IR spectrum, ν, cm–1: 585, 699, 730, 830, 945, 1150 m (SO2), 1189, 1233, 1352 s (SO2), 1508, 2820, 3082. 1Н NMR spectrum (DMSO-d6), δ, ppm: 3.12–3.14 m (4Н, С3Н2, С5Н2), 3.32–3.34 m (4Н, С2Н2, С6Н2), 6.94–6.98 m (2Н, С2′′H, С6′′H), 7.04–7.08 m (2Н, С3′′H, С5′′H), 7.48–7.49 m (3Н, 5′-Ph), 7.56–7.59 m (2Н, 5′-Ph), 9.28 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 46.1 (2C, SO2NCH2), 49.1 (2C, ArNCH2), 115.4 d (2С, С3′′, С5′′, 2JCF 23.0 Hz), 118.1 d (2С, С2′′, С6′′, 3JCF 7.5 Hz), 128.3 (2С), 128.4, 129.5, 130.2 (2С), 143.4 d (С1′′, 4JCF 3.5 Hz), 145.6, 147.5 (С4′), 154.8 (С2′), 156.5 d (С4′′, 1JCF 237.0 Hz). Mass spectrum, m/z (Irel, %): 404.3 (100) [M + H]+. Found, %: С 56.62; Н 4.47; N 10.39; S 15.93. C19H18FN3O2S2. Calculated, %: С 56.56; Н 4.50; N 10.41; S 15.89.
Ethyl 4-[(5-phenyl-1,3-thiazol-4-yl)sulfonyl]-1-piperazinecarboxylate (14k). Yield 2.90 g (76%), colorless crystals, mp 109–111°С (EtOH). IR spectrum, ν, cm–1: 588, 723, 950, 1150 s (SO2), 1253 s (C–O), 1353 s (SO2), 1422, 1440, 1696 s (C=O), 2869, 3093. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.17 t (3Н, ОСН2СН3, 3JНН 7.5 Hz), 3.19–3.22 m (4Н, С3Н2, С5Н2), 3.42–3.45 m (4Н, С2Н2, С6Н2), 4.02 q (2Н,ОСН2СН3, 3JНН 7.5 Hz), 7.47–7.49 m (3Н, 5′-Ph), 7.55–7.56 m (2Н, 5′-Ph), 9.26 s (1Н, С2′Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 14.6 (CH3), 46.0 (2C, SO2NCH2), 49.1 (OCH2), 61.1 (2C, CO-NCH2), 128.3 (2C), 129.6, 129.7, 130.2 (3C), 154.4, 154.9 (C2′), 165.2 (CO). Mass spectrum, m/z (Irel, %): 382.3 (100) [M + H]+. Found, %: С 50.44; Н 4.98; N 11.03; S 16.83. C16H19N3O4S2. Calculated, %: С 50.38; Н 5.02; N 11.02; S 16.81.
N-(1,1-Dioxotetrahydrothiophen3-yl)-N-(4-methylbenzyl)-5-phenyl-1,3-thiazole-4-sulfonamide (15a). Yield 3.56 g (77%), colorless crystals, mp 160–162°С (EtOH). IR spectrum, ν, cm–1: 598, 753, 1113 s (SO2), 1139 s (SO2), 1315 s (SO2),1346 s (SO2), 1426, 3011. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.99–2.04 m (1Н, O2SC2′HAHB), 2.13–2.16 m (1Н, O2SC2′HAHB), 2.27 s (3Н, СН3), 3.02–3.16 m (4Н, O2SC5′H2С4′Н2), 4.42 d (1Н, NCHAHB, 2JНН 18.0 Hz), 4.56 d (1Н, NCHAHB, 2JНН 18.0 Hz), 4.74–4.76 m (1Н, O2SC2′H2С3′Н), 7.13 d (2Н, С2′′Н, С6′′Н, 3JНН 8.0 Hz), 7.21 d (2Н, С3′′Н, С5′′Н, 3JНН 8.0 Hz), 7.46–7.49 m (3Н, 5-Ph), 7.53–7.55 m (2Н, 5-Ph), 9.26 s (1Н, С2Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 20.7 (CH3), 27.5 (C4′H2), 47.7 (C5′H2), 50.4 (C2′H2), 51.4 (C3′H), 54.1 (NCH2), 127.0 (2C), 128.3 (3C), 129.0 (3C), 129.6, 130.2 (3C), 135.2, 136.5, 155.1 (C2). Mass spectrum, m/z (Irel, %): 463.5 (100) [M + H]+. Found, %: С 54.58; Н 4.74; N 6.04; S 20.83. C21H22N2O4S3. Calculated, %: С 54.52; Н 4.79; N 6.06; S 20.79.
N-(1,1-Dioxotetrahydrothiophen-3-yl)-N-(4-methoxybenzyl)-5-phenyl-1,3-thiazole-4sulfonamide (15b). Yield 3.54 g (74%), colorless crystals, mp 147–149°С (EtOH). IR spectrum, ν, cm–1: 598, 753, 944, 1035, 1112 m (SO2), 1138 s (SO2), 1314 s (SO2), 1345 s (SO2), 1515, 2959, 3008. 1Н NMR spectrum (DMSO-d6), δ, ppm: 2.00–2.05 m (1Н, O2SC2′HAHB), 2.12–2.16 m (1Н, O2SC2′HAHB), 3.03–3.17 m (4Н, O2SC5′H2С4′Н2), 3.73 s (3Н, ОСН3), 4.39 d (1Н, NCHAHB, 2JНН 16.0 Hz), 4.53 d (1Н, NCHAHB, 2JНН 16.0 Hz), 4.73–4.77 m (1Н, O2SC2′H2С3′Н), 6.89 d (2Н, С2′′Н, С6′′Н, 3JНН 8.0 Hz), 7.25 d (2Н, С3′′Н, С5′′Н, 3JНН 8.0 Hz), 7.46–7.48 m (3Н, 5-Ph), 7.53–7.56 m (2Н, 5-Ph), 9.29 s (1Н, С2Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 27.5 (C4′H2), 47.5 (C5′H2), 50.5 (C2′H2), 51.4 (C3′H), 54.1 (NCH2), 55.1 (OCH3), 113.9 (2C), 128.3 (2C), 128,4 (2C), 129.6, 129.9, 130.2 (2C), 143.0, 144.9, 146.5, 155.1 (C2), 158.5 (C4′′-OCH3). Mass spectrum, m/z (Irel, %): 479.4 (100) [M + H]+. Found, %: С 52.79; Н 4.60; N 5.89; S 20.11. C21H22N2O5S3. Calculated, %: С 52.70; Н 4.63; N 5.85; S 20.10.
N-(1,1-Dioxotetrahydrothiophen-3-yl)-N-(4-ethoxybenzyl)-5-phenyl-1,3-thiazole-4-sulfonamide (15c). Yield 3.60 g (73%), colorless crystals, mp 171–173°С (EtOH). IR spectrum, ν, cm–1: 598, 753, 845, 1043, 1113 s (SO2), 1140 s (SO2), 1177, 1253 s (C–O), 1290, 1318 s (SO2), 1349 s (SO2), 1515, 1613, 2975, 3081. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.31 t (3Н,ОСН2СН3, 3JНН 7.5 Hz), 1.99–2.05 m (1Н, O2SC2′HAHB), 2.12–2.16 m (1Н, O2SC2′HAHB), 3.02–3.17 m (4Н, O2SC5′H2С4′Н2), 3.99 q (2Н,ОСН2СН3, 3JНН 7.5 Hz), 4.39 d (1Н, NCHAHB, 2JНН 18.0 Hz), 4.52 d (1Н, NCHAHB, 2JНН 18.0 Hz), 4.72–4.77 m (1Н, O2SC2′H2С3′Н), 6.87 d (2Н, С2′′Н, С6′′Н, 3JНН 8.0 Hz), 7.23 d (2Н, С3′′Н, С5′′Н, 3JНН 8.0 Hz), 7.46–7.48 m (3Н, 5-Ph), 7.53–7.54 m (2Н, 5-Ph), 9.29 s (1Н, С2Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 14.7 (CH3), 27.5 (C4′H2), 47.5 (C5′H2), 50.5 (C2′H2), 51.5 (C3′H), 54.1 (NCH2), 63.0 (OCH2), 114.3 (2C), 128.3 (2C), 128,4 (2C), 129.6, 129.7, 130.2 (2C), 143.0, 146.5, 149.3, 155.0 (C2), 157.8 (C4′′-OEt). Mass spectrum, m/z (Irel, %): 493.5 (100) [M + H]+. Found, %: С 53.66; Н 4.87; N 5.65; S 19.58. C22H24N2O5S3. Calculated, %: С 53.64; Н 4.91; N 5.69; S 19.53.
N-(1,1-Dioxotetrahydrothiophen-3-yl)-N-(4-fluorobenzyl)-5-phenyl-1,3-thiazole-4-sulfonamide (15d). Yield 3.31 g (71%), colorless crystals, mp 115–117°С (EtOH). IR spectrum, ν, cm–1: 598, 751, 844, 1034, 1114 s (SO2), 1138 s (SO2), 1230, 1313 s (SO2),1347 s (SO2), 1430, 1510, 1605, 2957, 3010. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.93–2.04 m (1Н, O2SC2′HAHB), 2.13–2.20 m (1Н, O2SC2′HAHB), 3.04–3.21 m (4Н, O2SC5′H2С4′Н2), 4.47 d (1Н, NCHAHB, 2JНН 18.0 Hz), 4.60 d (1Н, NCHAHB, 2JНН 18.0 Hz), 4.73–4.82 m (1Н, O2SC2′H2С3′Н), 7.14–7.18 m (2Н, С2′′Н, С6′′Н), 7.35–7.38 m (2Н, С3′′Н, С5′′Н), 7.47–7.48 m (3Н, 5-Ph), 7.53–7.54 m (2Н, 5-Ph), 9.29 s (1Н, С2Н). 13С NMR spectrum (DMSO-d6), δC, ppm: 27.5 (C4′H2), 47.2 (C5′H2), 50.4 (C2′H2), 51.3 (C3′H), 54.1 (NCH2), 115.2 d (2С, С3′′, С5′′, 2JCF 21.4 Hz), 128.2, 128.3 (2C), 129.0 d (2С, С2′′, С6′′, 3JCF 8.8 Hz), 129.7, 130.2 (2C), 134.4, 143.2, 146.3, 155.1 (С2), 160.3 d (С4′′, 1JCF 238.0 Hz). Mass spectrum, m/z (Irel, %): 467.2 (100) [M + H]+. Found, %: С 51.54; Н 4.07; N 5.97; S 20.68. C20H19FN2O4S3. Calculated, %: С 51.48; Н 4.10; N 6.00; S 20.62.
Study of antitumor activity. The methodology of in vitro antitumor screening, as well as the rules for data interpretation, are described in detail on the NCI Development Therapeutics Program website [28].
REFERENCES
Cao, B., Soerjomataram, I., and Bray, F., in: World Cancer Report: Cancer Research for Cancer Prevention, Wild, C.P., Weiderpass, E., and Stewart, B.W., Eds., Lyon: International Agency for Research on Cancer, 2020.
Ramos-Inza, S., Aydillo, C., Sanmartín, C., and Plano, D., in Heterocycles – Synthesis and Biological Activities, Nandeshwarappa, B.P., Ed., IntechOpen, 2020. https://doi.org/10.5772/intechopen.82741
Alizadeh, S.R. and Hashemi, S.M., Med. Chem. Res., 2021, vol. 30, no. 4, p. 771. https://doi.org/10.1007/s00044-020-02686-2
Petrou, A., Fesatidou, M., and Geronikaki, A., Molecules, 2021, vol. 26, no. 11, p. 3166. https://doi.org/10.3390/molecules26113166
Wang, Y.-J., Patel, B.A., Anreddy, N., Zhang, Y.-K., Zhang, G.-N., Alqahtani, S., Singh, S., Shukla, S., Kaddoumi, A., Ambudkar, S.V., Talele, T.T., and Chen, Z.-S., Sci. Rep., 2017, vol. 7, article 42106. https://doi.org/10.1038/srep42106
Lu, H., Rogowskyj, J., Yu, W., Venkatesh, A., Khan, N., Nakagawa, S., Goosssens, N., Koh, A.P., Higashi, T., Gunasekaran, G., Schwarz, M.E., Hiotis, S.P., Xu, X., Kinney, W., Hoshida, Y., Block, T., Cuconati, A., and Du, Y., Bioorg. Med. Chem. Lett., 2016, vol. 26, no. 23, p. 5819. https://doi.org/10.1016/j.bmcl.2016.10.015
Chen, C., Song, J., Wang, J., Xu, C., Chen, C., Gu, W., Sun, H., and Wen, X., Bioorg. Med. Chem. Lett., 2017, vol. 27, no. 4, p. 845. https://doi.org/10.1016/j.bmcl.2017.01.018
Rakesh, K.P., Wang, S.M., Leng, J., Ravindar, L., Asiri, A.M., Marwani, H.M., and Qin, H.L., Anticancer Agents Med. Chem., 2018, vol. 18, no. 4, p. 488. https://doi.org/10.2174/1871520617666171103140749
Zhao, C., Rakesh, K.P., Ravidar, L., Fang, W.-Y., and Qin, H.-L., Eur. J. Med. Chem., 2019, vol. 162, p. 679. https://doi.org/10.1016/j.ejmech.2018.11.017
Yurttaş, L., Çiftçi, G.A., and Temel, H.E., Turk. J. Biochem., 2021, vol. 46, no. 5, p. 533. https://doi.org/10.1515/tjb-2020-0238
Wang, Y., Fu, J., Sun, Y., Wu, G., Lu, A., Zhang, S., Goodnow, R., Gilmer, T., Kastan, M., and Kirsch, D., Pat. WO 2019201283, 2019.
Dong, J., Huang, J., Zhou, J., Tan, Y., Jin, J., Tan, X., Wang, B., Yu, T., Wu, C., Chen, S., and Wang, T.-L., ACS Med. Chem. Lett., 2020, vol. 11, no. 7, p. 1463. https://doi.org/10.1021/acsmedchemlett.0c00239
Shim, E.K., Kim, N.D., Shim, T.B., and Kim, S.Y., Patent EP 2647637, 2013.
Young, R. and Bosc, D., Patent WO 2017027984, 2017.
Kornienko, A.N., Pil’o, S.G., Prokopenko, V.M., and Brovarets, V.S., Russ. J. Gen. Chem., 2014, vol. 84, no. 4, p. 686. https://doi.org/10.1134/S1070363214040148
Demydchuk, B.A., Kondratyuk, K.M., Korniyenko, A.N., Brovarets, V.S., Vasylyshyn, R.Y., Tolmachev, A., and Lukin, O., Synth. Commun., 2012, vol. 42, no. 19, p. 2866. https://doi.org/10.1080/00397911.2011.571356
Belyuga, A.G., Brovarets, V.S., and Drach, B.S., Russ. J. Gen. Chem., 2004, vol. 74, no. 9, p. 1418. https://doi.org/10.1007/s11176-005-0024-5
Mori, M., Stelitano, G., Chiarelli, L.R., Cazzaniga, G., Gelain, A., Barlocco, D., Pini, E., Meneghetti, F., and Villa, S., Pharmaceuticals, 2021, vol. 14, no. 2, p. 155. https://doi.org/10.3390/ph14020155
Matthies, D., Bartsch, B., and Richter, H., Arch. Pharm., 1981, vol. 314, no. 3, p. 209. https://doi.org/10.1002/ardp.19813140305
Skulnick, H.I., Johnson, P.D., Aristoff, P.A., Morris, J.K., Lovasz, K.D., Howe, W.J., Watenpaugh, K.D., Janakiraman, M.N., Anderson, D.J., and Reischer, R., J. Med. Chem., 1997, vol. 40, no. 7, p. 1149. https://doi.org/10.1021/jm960441m
Hay, J.V. and Wolf, A.D., Patent US 4943312, 1990.
Kachaeva, M.V., Pilyo, S.G., Demydchuk, B.A., Prokopenko, V.M., Zhirnov, V.V., and Brovarets, V.S., Int. J. Curr. Res., 2018, vol. 10, no. 5, p. 69410.
Pilyo, S.G., Kozachenko, О.P., Zhirnov, V.V., Kachaeva, M.V., Kobzar, O.L., Vovk, A.I., and Brovarets, V.S., Ukr. Bioorg. Acta, 2020, vol. 15, no. 2, p. 13. https://doi.org/10.15407/bioorganica2020.02.013
Alley, M.C., Scudiero, D.S., Monks, P.A., Hursey, M.L., Czerwinski, M.J., Fine, D.L., Abbott, B.J., Mayo, J.G., Shoemaker, R.H., and Boyd, M.R., Cancer Res., 1988, vol. 48, no. 3, p. 589.
Grever, M.R., Scudiero, R.H., and Chabner, B.A., Semin. Oncol., 1992, vol. 19, no. 6, p. 622.
Boyd, M.R. and Paull, K.D., Drug Develop. Res., 1995, vol. 34, no. 2, p. 91. https://doi.org/10.1002/ddr.430340203
Shoemaker, R.H., Nature Rev., 2006, vol. 6, no. 10, p. 813. https://doi.org/10.1038/nrc1951
NCI-60 Human Tumor Cell Lines Screen. DTP Developmental Therapeutics Program, NIH website, March 20, 2020. https://dtp.cancer.gov/discovery_development/nci-60/default.htm
ACKNOWLEDGMENTS
The authors are grateful to the National Cancer Institute (USA) for the study of antitumor activity and Enamine Ltd. for material and technical support.
Funding
The work was financially supported by the National Research Foundation of Ukraine (competition “Science for the Security of Man and Society,” no. 2020.01/0075).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Severin, A.O., Pilyo, S.G., Potikha, L.M. et al. Synthesis and Antitumor Activity of 5-Phenyl-1,3-thiazole-4-sulfonamide Derivatives. Russ J Gen Chem 92, 174–184 (2022). https://doi.org/10.1134/S1070363222020062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222020062