Abstract
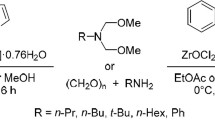
A series of bi- and trinuclear pyrrole derivatives was prepared by the 5 mol % ZrOCl2 ·8H2O-catalyzed CH-sp2-aminomethylation reaction of pyrrole with bis(1,3-oxazacycloalk-3-yl)methanes. The reaction proceeds at the positions 2, 5 or 2 of pyrrole depending on the amount of aminomethylating reagents obtained from formaldehyde and α,ω-amino alcohols (2-aminoethan-1-ol, 3-aminopropan-1-ol, 4-aminobutan-1-ol). The aminomethylation reaction of indole with bis(1,3-oxazolidin-3-yl)methane proceeds at the position 3. In the case of carbazole, N-aminomethylation proceeds under these conditions.
Similar content being viewed by others
References
RF Patent 2384574, 2008.
Willy, M.E., Manda, B., Shatin, D., Drinkard, C.R., and Graham, D.J., J. Am. Acad. Child Adolesc. Psychiatr., 2002, vol. 41. no. 7, p. 785. https://doi.org/10.1097/00004583-200207000-00009
Gaine, S.P., Rubin, L.J., Kmetzo, J.J., Palevsky, H.I., and Traill, T.A., CHEST, 2000, vol. 118, no. 5, p. 1496. https://doi.org/10.1378/chest.118.5.1496
Kankaanpää, A., Ellermaa, S., Meririnne, E., Hirsjärvi, P., and Seppälä, T., J. Pharm. Exp. Ther., 2002, vol. 300, no. 2, p. 450. https://doi.org/10.1124/jpet.300.2.450
Katritzky, A.R., Singh, S.K., and Bobrov, S., J. Org. Chem., 2004, vol. 69, no. 26, p. 9313. https://doi.org/10.1021/jo0485334
Maksimov, V., Zaynullin, R., Akhmadiev, N., Segura-Ceniceros, E. P., Martinez Hernandez, J.L., Bikbulatova, E., Akhmetova, V., Kunakova, R., Ramos, R., and Ilyina, A., Med. Chem. Res., 2016, vol. 25, p. 1384. https://doi.org/10.1007/s00044-016-1574-2
Sokolov, V.B., Makhaeva, G.F., Aksinenko, A.Yu., Grigoriev, V.V., Shevtsova, E.F., and Bachurin, S.O., Russ. Chem. Bull., 2017, vol. 66, no. 10, p. 1821. https://doi.org/10.1021/jo0485334
Akhmetova, V.R., Bikbulatova, E.M., Akhmadiev, N.S., Yanybin, V.M., Boiko, T.F., Kunakova, R.V., and Ibragimov, A.G., Russ. J. Org. Chem., 2018, vol. 54, no. 5, p. 701. https://doi.org/10.1134/S1070428018050056
Salas-Coronado, R., Gálvez-Ruiz, J.C., Guadarrama-Pérez, C., and Flores-Parr, A., Heterocycles, 2003, vol. 60, no. 5, p. 1111. https://doi.org/10.3987/COM-03-9713
Khabibullina, G.P., Yanybin, V.M., Ibragimov, A.G., and Dzhemilev, U.M., Chem. Heterocycl. Compd., 2014, vol. 50, p. 726. https://doi.org/10.1007/s10593-014-1527-y
Akhmetova, V.R., Bikbulatova, E.M., Akhmadiev, N.S., Galimzyanova, N.F., Kunakova, R.V., and Ibragimov, A.G., Chem. Heterocycl. Compd., 2018, vol. 54, no. 5, p. 520. https://doi.org/10.1007/s10593-018-2299-6
Funding
The authors are grateful to the Center for Collective Use “Agidel” of Institute of Petrochemistry and Catalysis of the Ufa Federal Research Center of the Russian Academy of Sciences for structural studies of compounds, as well as N.F. Galimzyanova for the study of fungicidal activity.
This work was financially supported by the Russian Foundation for Basic Research (project no. 17-43-020292p_a) and the Academy of Sciences of the Republic of Bashkortostan in the frame of the project part of the governmental task (no. AAAA-A19-119022290010-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Russian Text © The Author(s), 2019, published in Zhurnal Obshchei Khimii, 2019, Vol. 89, No. 9, pp. 1346–1351.
Rights and permissions
About this article
Cite this article
Akhmetova, V.R., Bikbulatova, E.M., Kunakova, R.V. et al. Skeletal Diversity in Catalytic Synthesis of (1,3-Oxazacycloalk-3-ylmethyl)-Substituted Pyrroles. Russ J Gen Chem 89, 1760–1764 (2019). https://doi.org/10.1134/S1070363219090056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363219090056