Abstract
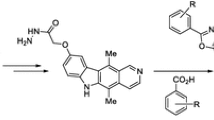
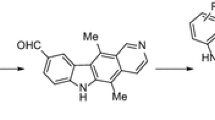
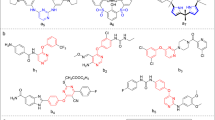
A series of ellipticine derivatives 8a–8j containing oxadiazole structural block are synthesized and evaluated for their anticancer activity on human cancer cell lines (Colo-205, MCF-7, A-549, and KB). Among those, the products 8b, 8c, 8d, 8i exhibit potent cytotoxic activity with GI50 values ranging from <0.1 to 23.6 µM. The positive control etoposide GI50 values are ranging from 0.13 to 3.08 µM.
Similar content being viewed by others
References
Goodwin, S., Smith, A.F., and Horning, E.C., J. Am. Chem. Soc., 1959, vol. 81, p. 1903. doi https://doi.org/10.1021/ja01517a031
Stiborová, M., Bieler, C. A., Wiessler, M., and Frei, E., Biochem. Pharmacol., 2001, vol. 62, p. 1675. doi https://doi.org/10.1016/S0006-2952(01)00806-1
Stiborová, M., Rupertová, M., Schmeiser, H.H., and Frei, E., Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub., 2006, vol. 150, p. 13.
Stiborová, M., Rupertovâ, M., and Frei, E., Biochim. Biophys. Acta, 2011, vol. 1814, p. 175. doi https://doi.org/10.1016/j.bbapap.2010.05.016
Auclair, C., Arch. Biochem. Biophys., 1987, vol. 259, p. 1. doi https://doi.org/10.1016/0003-9861(87)90463-2
Singh, M.P., Hill, G.C., Peoch, D., Rayner, B., Inabach, J.L., and Lown, J.W., Biochemistry, 1994, vol. 33, p. 10271. doi https://doi.org/10.1021/bi00200a007
Chu, Y. and Hsu, M.T. Nucleic Acids Res., 1992, vol. 20, p. 4033. doi https://doi.org/10.1093/nar/20.15.4033
Monnot, M., Mauffret, O., Simon, V., Lescot, E., Psaume, B., Saucier, J.M., Charra, M., Belehradek, J., Jr., and Fermandjian, S., J. Biol. Chem., 1991, vol. 25, p. 1820.
Fossé, P., René, B., Charra, M., Paoletti, C., and Saucier, J.M., Mol. Pharmacol., 1992, vol. 42, p. 590.
Woodward, R.B., Iacobucci, G.A., and Hochstein, F.A., J. Am. Chem. Soc., 1959, vol. 81, p. 4434. doi https://doi.org/10.1021/ja01525a085
Garbett, N.C. and Graves, D.E., Curr. Med. Chem., 2004, vol. 4, p. 149
Stiborova, M., Rupertova, M.S., Dohalska, M.B., Weissler, M., and Frei, E., Chem. Res. Toxicol., 2003, vol. 16, p. 38. doi https://doi.org/10.1021/tx0200818
Malleshappa, N.N. and Harun, M.P., Eur. J. Med. Chem., 2012, vol. 56, p. 56. doi https://doi.org/10.1016/j.ejmech.2012.08.012.
Asati, V., Mahapatra, D.K., and Bharti, S.K., Eur. J. Med. Chem., 2014, vol. 87, p. 814. doi https://doi.org/10.1016/j.ejmech.2014.10.025
Farshori, N.N., Banday, M.R., Ahmad, A., Khan, A.U., and Rauf, A., Bioorg. Med. Chem. Lett., 2010, vol. 20, p. 1933. doi https://doi.org/10.1016/j.bmcl.2010.01.126
Cui, Z.N., Shi, Y.X., Zhang, L., Ling, Y., Li, B.J., Nishida, Y., and Yang, X.L., J. Agric. Food Chem., 2012, vol. 60, p. 11649. doi https://doi.org/10.1021/jf303807a
Li, Y.H., Zhu, H.J., Chen, K., Liu, R., Khallaf, A., Zhang, X.N., and Ni, J.P., Org. Biomol. Chem., 2013, vol. 11, p. 3979. doi https://doi.org/10.1039/C3OB40345A
El-Emam, A.A., Al-Deeb, O.A., Al-Omar, M., and Lehmann, J., Bioorg. Med. Chem. 2004, vol. 12, p. 5107. doi https://doi.org/10.1016/j.bmc.2004.07.033
Jayashankar, B., Lokanathrai, K.M., Baskaran, N., and Sathish, H.S., Eur. J. Med. Chem., 2009, vol. 44, p. 3898. doi https://doi.org/10.1016/j.ejmech.2009.04.006
Kucukguzel, S.G., Oruc, E.E., Rollas, S., Sahin, F., and Ozbek, A., Eur. J. Med. Chem., 2002, vol. 37, p. 197.
Bondock, S., Adel, S., Etman, H.A., and Badria, F.A., Eur. J. Med. Chem., 2012, vol. 48, p. 192. doi https://doi.org/10.1016/j.ejmech.2011.12.013
James, N.D. and Growcott, J.W., Drugs Future, 2009, vol. 34, p. 624. doi https://doi.org/10.1358/dof.2009.34.8.1400202.624
Skehn, P., Storeng, R., Scudiero, A., Monks, J., McMohan, D., Vistica, D., Jonathan, W.T., Bokesch, H., Kenney, S., and Boyd, R.M., J. Natl. Cancer Inst., 1990, vol. 82, p. 1107.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sumalatha, S., Namrata, V., Lakshmi, M. et al. Synthesis and Anticancer Activity of Oxadiazole Incorporated Ellipticine Derivatives. Russ J Gen Chem 89, 505–510 (2019). https://doi.org/10.1134/S107036321903023X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036321903023X