Abstract
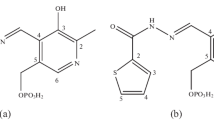
Composition of coordination compounds formed by copper(II) or zinc(II) ions and pyridine-2-, -3-, and -4-carbohydrazones of pyridoxal-5-phosphate in aqueous solution at 298.2 K, рН 7.4, I 0.25 has been studied by means of spectrophotometry. Conditional constants of the complexes stability have been determined. Cu(II) ions formed more stable ML2 coordination compounds in comparison with Zn(II), zinc(II) ions are efficiently bound to the derivative of picolinic acid hydrazide to form the ML complex.
Similar content being viewed by others
References
Ala, A., Walker, A.P., Ashkan, K., Dooley, J.S., and Schilsky, M.L., Lancet, 2007, vol. 369, p. 397. doi 10.1016/S0140-6736(07)60196-2
Gamov, G.A., Kiselev, A.N., Aleksandriiskii, V.V., and Sharnin, V.A., J. Mol. Liq., 2017, vol. 242, p. 1148. doi 10.1016/j.molliq.2017.07.106
Brittenham, G.M., Ann. NY Acad. Sci., 1990, vol. 612, p. 315. doi 10.1111/j.1749-6632.1990.tb24319.x
Hermes-Lima, M., Gonçalves, M.S., and Andrade, R.G., Jr., Mol. Cell. Biochem., 2001, vol. 228, p. 73. doi 10.1023/A:1013348005312
Rao, T.R. and Singh, G., Cryst. Res. Technol., 1989, vol. 24, no. 10, p. 169. doi 10.1002/crat.2170241019
Richardson, D.R. and Ponka, P., J. Lab. Clin. Med., 1998, vol. 131, no. 4, p. 306. doi 10.1016/S0022-2143 (98)90180-9
Saito, S., Okabe, M., Yoshida, K., and Kurasaki, M., Pharmacol. Toxicol., 1999, vol. 84, p. 255. doi 10.1111/j.1600-0773.1999.tb01491.x
Richardson, D.R., Hefter, G.T., May, P.M., Webb, J., and Baker, E., Biol. Metals, 1989, vol. 2, p. 161. doi 10.1007/BF01142555
Murphy, T.B., Johnson, D.K., Rose, N.J., Aruffo, A., and Schomaker, V., Inorg. Chim. Acta (C), 1982, vol. 66, p. L67. doi 10.1016/S0020-1693(00)85778-3
Richardson, D.R. and Bernhardt, P.V., J. Biol. Inorg. Chem., 1999, vol. 4, no. 3, p. 266. doi 10.1007/s007750050312
Mezey, R.-S., Máthé, I., Shova, S., Grecu, M.-N., and Rosu, T., Polyhedron, 2015, vol. 102, p. 684. doi 10.1016/j.poly.2015.10.035
Wis Vitolo, L.M., Hefter, G.T., Clare, B.W., and Webb, J., Inorg. Chim. Acta, 1990, vol. 170, no. 2, p. 171. doi 10.1016/S0020-1693(00)80472-7
Filipsky, T., Ríha, M., Hrdina, R., Vávrová, K., and Mladenka, P., Bioorg. Chem., 2013, vol. 49, p. 1. doi 10.1016/j.bioorg.2013.06.002
Powell, K.J., Brown, P.L., Byrne, R.H., Gajda, T., Hefter, G., Sjöberg, S., and Wanner, S., Pure Appl. Chem., 2005, vol. 77, no. 4, p. 739. doi 10.1351/pac200577040739
Brown, P.L. and Ekberg, C., Hydrolysis of Metal Ions, Weinheim: Wiley-VCH Verlag GmbH & Co, 2016.
Powell, K.J., Brown, P.L., Byrne, R.H., Gajda, T., Hefter, G., Sjöberg, S., and Wanner, H., Pure Appl. Chem., 2007, vol. 79, no. 5, p. 895. doi 10.1351/pac200779050895
Powell, K.J., Brown, P.L., Byrne, R.H., Gajda, T., Hefter, G., Leuz, A -K., Sjöberg, S., and Wanner, H., Pure Appl. Chem., 2013, vol. 85, no. 12, p. 2249. doi 10.1351/PAC-REP-13-06-03
Gridchin, S.N., Kochergina, L.A., and Konovalov, P.G., Russ. J. Coord. Chem., 2003, vol. 29, no. 12, p. 868. doi 10.1023/B:RUCO.0000008399.53700.43
Kitaev, Yu.P. and Buzykin, B.I., Gidrazony (Hydrazones), Moscow: Nauka, 1974, p.105.
De Stefano, C., Foti, C., Giuffrü, O., and Milea, D., New, J. Chem., 2016, vol. 40, p. 1443. doi 10.1039/C5NJ02531A
Mouat, M.F. amd Manchester, K.L., Comp. Haematol. Int., 1998, vol. 8, no. 1, p. 58. doi 10.1007/BF02628107
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.A. Gamov, M.N. Zavalishin, A.Yu. Khokhlova, A.V. Gashnikova, V.A. Sharnin, 2018, published in Zhurnal Obshchei Khimii, 2018, Vol. 88, No. 7, pp. 1144–1148.
Rights and permissions
About this article
Cite this article
Gamov, G.A., Zavalishin, M.N., Khokhlova, A.Y. et al. Stability of Cu(II) and Zn(II) Complexes with Pyridinecarbohydrazones of Pyridoxal-5-phosphate in Aqueous Solution. Russ J Gen Chem 88, 1436–1440 (2018). https://doi.org/10.1134/S1070363218070149
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363218070149