Abstract
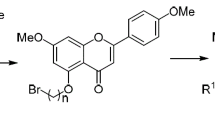
A number of 4'-substituted (R = H, Me, Cl, F) flavone derivatives is synthesized from 2-hydroxyacetophenones using the modified Baker–Venkataraman reaction. Compound [3-(4-fluorobenzoyl)-5- hydroxy-4'-fluoroflavone] was synthesized for the first time with the yield of 12%. Antiproliferative assays indicate that the synthesized flavones with F substituent at the 4' position demonstrate higher activity than the other flavone derivatives, particularly against HeLa and MCF-7 with the IC50 9.5 and 2.7 μM, respectively.
Similar content being viewed by others
References
Cabrera, M., Simoens, M., Falchi, G., Lavaggi, M.L., Piro, O.E., Castellano, E.E, Vidal, A., Azqueta, A., Monge, A., Lopez de Cerian, A., Sagrera, G., Seoane, G., Cerecetto, H., and Gonzalez, M., Bioorg. Med. Chem., 2007, vol. 15, p. 3356. doi 10.1016/j.bmc.2007.03.031
Mughal, E.U., Ayaz, M., Hussain, Z., Hasan, A., Sadiq, A., Riaz, M., Malik, A., Hussain, S., and Choudhary, M.I., Bioorg. Med. Chem., 2006, vol. 14, p. 4704. doi 10.1016/j.bmc.2006.03.031
Shenvi, S., Kumar, K., Hatti, KS., Rijesh, K., Diwakar, L., and Reddy, G.C., Eur. J. Med. Chem., 2013, vol. 62, p. 435. doi 10.1016/j.ejmech.2013.01.018
Liu, H.C., Dong, A.J., Gao, C.M., Tan, C.Y., Xie, Z.H., Zu, X.Y., Qu, L., and Jiang, Y.Y., Bioorg. Med. Chem., 2010, vol. 18, p. 6322. doi 10.1016/j.bmc.2010.07.019
Baker, W., J. Chem. Soc., 1933, vol. 8, p. 1381. doi 10.1039/JR9330001381
Ganguly, A.K., Kaur, S., Mahata, P.K., Biswas, D., Pramanik, B.N., and Chan, T.M., Tetrahedron Lett., 2005, vol. 46, no. 4, p. 4119. doi 10.1016/j.tetlet.2005.04.010
Ganguly, A.K., Mahata, P.K., and Biswas, D., Tetrahedron Lett., 2006, vol. 47, no. 12, p. 1347. doi 10.1016/j.tetlet.2005.12.062
Bois, F., Beney, C., Mariotte, A.M., and Boumendjel, A., Synlett, 1999, vol. 11, no. 9, p. 1480. doi 10.1055/s-1999-2844
Chee, C.F., Buckle, M.J.C., and Rahman, N.A., Tetrahedron Lett., 2011, vol. 52, no. 4, p. 3120. doi 10.1016/j.tetlet.2011.04.022
Franco, C., Rossella, F., Adriana, B., Paola, C., Daniela, S., and Francesca, R., Bioorg. Med. Chem., 2010, vol. 18, no. 3, p. 1273. doi 10.1016/j. bmc.2009.12.029
Jae, I.L., Hwa, S.S., and Mi, G.J., Bull. Korean Chem. Soc., 2005, vol. 26, no. 9, p. 1461. doi 10.1002/chin.200607144
Jiraporn, U., Chanpen, W., Weerasak, S., Patcharawee, N., and Narumol, P., J. Mol. Struct., 2011, vol. 1001, nos. 1–3, p. 152. doi 10.1016/j. molstruc.2011.06.035
Wang, X.J. and Liu, J.L., Chin. J. Org. Chem., 2014, vol. 34, no. 8, p. 1609. doi 10.6023/cjoc201402013
Masato, M., Masaki, T., Ayano, T., Takahide, T., Hiroto, T., and Takayuki, S., Chem. Pharm. Bull., 2010, vol. 58, no. 8, p. 1107. doi 10.1248/cpb.58.1107
Pinto, D.C.G.A., Silva Artur, M.S., Almeida Lucia, M.P.M., Cavaleiro Jose, A.S., and Elguero, J., Eur. J. Org. Chem., 2002, vol. 12, p. 3807. doi 10.1002/1099-0690 (200211)2002:22<3807::AID-EJOC3807>3.0.CO;2-2
Cardoso, A.M., Silva, A.M.S., Barros, C.M.F., Almeida, L.M.P.M., Ferrer-Correia, A.J., and Cavaleiro, J.A.S., J. Mass Spectrom., 1997, vol. 32, no. 9, p. 930. doi 10.1002/(SICI)1096-9888(199709)32:9<930::AIDJMS549> 3.0.CO;2-E
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, J. & Zhang, Y. An Efficient One-Pot Synthesis and Anticancer Activity of 4'-Substituted Flavonoids. Russ J Gen Chem 88, 1036–1041 (2018). https://doi.org/10.1134/S1070363218050328
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363218050328