Abstract
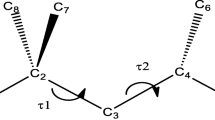
Quantum-chemical simulation of the ground state [the density function B3LYP/6-31G, B3LYP/6-31G(d), and B3LYP/6-31+G(d,p) and the perturbation theory MP2/6-31G(d) methods] and the transition states [the B3LYP/6-31G(d) method] of 4,4′-methoxypropylstilbene molecule has been performed. Using the Ellinger MM2 force field method, the potentials of internal rotation have been obtained for each rotational degree of freedom of the molecule. The B3LYP simulation has revealed the planarity of the conjugated system and the orthogonal position of the alkyl substituent, whereas the benzene rings have deviated by about 20° with respect to the double bond plane according to the MP2 data. Three transition states of the molecule corresponding to the saddle points of the first and the second orders have been revealed. The stationary points have been identified by means of vibrational analysis.
Similar content being viewed by others
References
Berezov, T.T. and Korovkin, B.F., Biologicheskaya khimiya (Biological Chemistry), Moscow: Meditsina, 1998.
Grebenkin, M.F. and Ivashhenko, A.V., Zhidkokristallicheskie materialy (Liquid Crystal Materials), Moscow: Khimiya, 1989.
Dashevskii, V.G., Konformatsionnyi analiz organicheskikh molekul (Conformational Analysis of Organic Molecules), Moscow: Khimiya, 1982, p. 15.
Aver’yanov, E.M., J. Struct. Chem., 2002, vol. 43, p. 360. doi 10.1023/A:1019673112979
Lhost, O. and Brédas, J.L., J. Chem. Phys., 1992, vol. 96, p. 5279. doi 10.1063/1.462713
Choi, C.H. and Kertesz, M., J. Phys. Chem. (A), 1997, vol. 101, p. 3823. doi 10.1021/jp970620v
Hofmann, H.J. and Birner, P., J. Mol. Struct., 1977, vol. 39, p. 145. doi 10.1016/0022-2860(77)85044-8
Momicchiolo, F., Baraldi, I., and Bruni, M.C., Chem. Phys., 1983, vol. 82, p. 229. doi 10.1016/0301-0104(83) 85359-2
Bally, T., Haselbach, E., Lanyiova, S., Marschner, F., and Rossi, M., Helv. Chim. Acta, 1976, vol. 459, p. 86. doi 10.1002/hlca.19760590215
Perrin, H. and Berges, G., THEOCHEM, 1981, vol. 76, p. 299. doi 10.1016/0166-1280(81)85006-3
Troe, J. and Weitzel, K.-M.J., J. Chem. Phys., 1988, vol. 88, p. 7030. doi 10.1063/1.454402
Galvao, D.S., Soos, Z.G., Ramasesha, S., and Etemad, S., J. Chem. Phys., 1993, vol. 98, p. 3016. doi 10.1063/1.464128
Kwasniewski, S.P., Claes, L., Francois, J.-P., and Deleuze, M.S., J. Chem. Phys., 2003, vol. 118, no. 17, p. 7823. doi 10.1063/1.1563617
Traetteberg, M., Frantsen, E.B., Mijlhoff, F.C., and Hoekstra, A., J. Mol. Struct., 1975, vol. 26, p. 57. doi 10.1016/0022-2860(75)80066-4
Potapov, V.M., Stereokhimiya (Stereochemistry), Moscow: Khimiya, 1976.
Burkert, U. and Allinger, N.L., Molecular Mechanics, Washington: American Chemical Society, 1982.
Internal Rotation in Molecules, Orville-Thomas, W.J., Ed., New York: Wiley-Interscience, 1974.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Vreven, Jr.T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian 03, Revision C.01, Gaussian, Inc., Wallingford CT, 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.K. Abulyaissova, S.O. Kenzhetaeva, M.S. Kasymova, 2017, published in Zhurnal Obshchei Khimii, 2017, Vol. 87, No. 6, pp. 902–909.
Rights and permissions
About this article
Cite this article
Abulyaissova, L.K., Kenzhetaeva, S.O. & Kasymova, M.S. Conformational space of 4,4′-methoxypropylstilbene molecule. Russ J Gen Chem 87, 1125–1131 (2017). https://doi.org/10.1134/S1070363217060044
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363217060044