Abstract
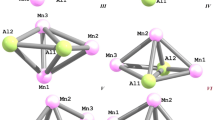
Geometric parameters of molecular structures of hexatomic heteronuclear (AlFe) clusters with various number of aluminum atoms (Al2Fe4 and Al3Fe3) were calculated using the density functional hybrid method in the OPBE/TZVP approximation. It was found that the first of these clusters is able to exist in nine and the second in twenty structural modifications substantially differing from each other in their stability and geometric parameters. The values of bond lengths, planar and dihedral (torsion) angles for some of these modifications are given.
Similar content being viewed by others
References
Dresvyannikov, A.F., Kolpakov, M.E., and Pronina, E.V., Russ. J. Gen. Chem., 2010, vol. 80, no. 10, p. 1901.
Dresvyannikov, A.F., Kolpakov, M.E., and Mironov, M.M., Inorg. Mater., 2012, vol. 3, no. 3, p. 193. doi 10.1134/S2075113311030075
Dresvyannikov, A.F. and Kolpakov, M.E., Russ. J. Appl. Chem., 2014, vol. 87, no. 2, p. 172. doi 10.1134/S1070427214020086
Dresvyannikov, A.F. and Kolpakov, M.E., Russ. J. Appl. Chem., 2014, vol. 87, no. 7, p. 867. doi 10.1134/S1070427214070040
Mikhailov, O.V. and Chachkov, D.V., Russ. J. Gen. Chem., 2016, vol. 86, no. 9, p. 1991. doi 10.1134/S1070363216090036
Schaefer, A., Horn, H., and Ahlrichs, R., J. Chem. Phys., 1992, vol. 97, no. 4, p. 2571. doi 10.1063/1.463096
Schaefer, A., Huber, C., and Ahlrichs, R., J. Chem. Phys., 1994, vol. 100, no. 8, p. 5829. doi 10.1063/1.467146
Hoe, W.-M., Cohen, A., and Handy, N.C., Chem. Phys. Lett., 2001, vol. 341, no. 1, p. 319. doi 10.1016/S0009-2614(01)00581-4
Perdew, J.P., Burke, K., and Ernzerhof, M., Phys. Rev. Lett., 1997, vol. 78, no. 7, p. 396. doi 10.1103/PhysRevLett.78.1396
Paulsen, H., Duelund, L., Winkler, H., Toftlund, H., and Trautwein, A.X., Inorg. Chem., 2001, vol. 40, no. 9, p. 2201. doi 10.1021/ic000954q
Swart, M., Groenhof, A.R., Ehlers, A.W., and Lammertsma, K., J. Phys. Chem. A, 2004, vol. 108, no. 25, p. 5479. doi 10.1021/jp049043i
Swart, M., Ehlers, A.W., and Lammertsma, K., Mol. Phys., 2004, vol. 102, no. 23, p. 2467. doi 10.1080/0026897042000275017
Swart, M., Inorg. Chim. Acta, 2007, vol. 360, no. 1, p. 179. doi 10.1016/j.ica.2006.07.073
Frisch, M.J, Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G.V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, H., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B.V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., and Fox, D.J., Gaussian 09, Revision A.01, Gaussian, Inc., Wallingford CT,2009.
Mikhailov, O.V. and Chachkov, D.V., Russ. J. Gen. Chem., 2014, vol. 84, no. 2, p. 329. doi 10.1134/S1070363214020297
Mikhailov, O.V. and Chachkov, D.V., Russ. J. Gen. Chem., 2014, vol. 84, no. 10, p. 1685. doi 10.1134/S1070363214100181
Mikhailov, O.V. and Chachkov, D.V., Russ. J. Gen. Chem., 2015, vol. 85, no. 3, p. 628. doi 10.1134/S1070363215030172
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D.V. Chachkov, O.V. Mikhailov, 2017, published in Zhurnal Obshchei Khimii, 2017, Vol. 87, No. 4, pp. 535–543.
Rights and permissions
About this article
Cite this article
Chachkov, D.V., Mikhailov, O.V. Molecular structure of hexatomic heteronuclear (AlFe) metal clusters as determined by the DFT quantum-chemical calculation. Russ J Gen Chem 87, 670–678 (2017). https://doi.org/10.1134/S1070363217040028
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363217040028