Abstract
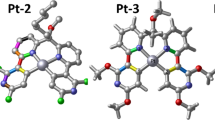
Two compounds 1-[(2-carboxymethyl)benzene]-3-[2-pyridine]triazene (HL) and 1-[(2-carboxymethyl) benzene]-3-[o-aminobenzoic acid]triazene (H2L') and two corresponding Pt(II) complexes, Pt(PPh3)2(L)Cl (1) and Pt(PPh3)2(L') (2), are theoretically studied by the density functional theory and time-dependent density functional theory. The geometric structure of complex 1 is optimized by B3LYP, PBE0, and M06 methods with the same mixed 6-31G(d)-LANL2DZ basis set. The absorption spectrum of complex 1 is simulated by the above method. As compared with the experimental data, the combination of M06/6-31G(d)-LANL2DZ and TD-M06/6-31G(d)-LANL2DZ is chosen for all other calculations including optimization of the ground-state and the lowest triplet excited state geometries, and the absorption and emission spectra. The detailed electronic transitions are analyzed to understand deeply the properties of spectra. Mobility of holes and electrons in 2 are studied computationally based on the Marcus theory. The ionization potential and electron affinity of complex 2 are calculated to evaluate qualitatively the hole- and electron-injection properties, respectively. Its potential as a dopant for phosphorescent OLEDs is explored.
Similar content being viewed by others
References
José, J., Escobar, N., Alvarado, C.C., Moreno, G.R., Morales, D.M., Walsh, P.J., and Hake, M.P., Inorg. Chem., 2007,vol. 46, no. 15, p. 6182. doi ?10.1021/ic700516p"10.1021/ic700516p
Vrieze, K. and Van, K.G., Comprehensive Coordination Chemistry,: Oxford Pergamon Press, 1987.
Kimball, D.B. and Haley, M.M., Angew. Chem. Int. Ed., 2002, vol. 41, no. 18, p. 3338. doi 10.1002/1521-3773 (20020916)41:18<3338::AID-ANIE3338>3.0.CO;2-7
Rouzer, C.A., Sabourin, M., Skinner, T.L., Thompson, E.J., Wood, T.O., Chmurny, G.N., Klose, J.R., Roman, J.M., Smith, R.H., and Michejda, C.J., Chem. Res. Toxicol., 1996, vol. 9, no. 1, p. 172. doi 10.1021/tx9500639
Nicolaou, K.C., Boddy, C.N.C., Li, H., Koumbis, A.E., Hughes, R., Natarajan, S., Jain, N.F., Ramanjulu, J.M., Bräse, S., and Solomon, M.E., Chem. Eur. J., 1999, vol. 5, no. 9, p. 2602. doi 10.1002/(SICI)1521-3765 (19990903)5:9<2602::AID-CHEM2602>3.0.CO;2-X
Bräse, S., Dahmen, S., and Pfefferkorn, M., J. Comb. Chem., 2000, vol. 2, no. 6, p. 710. doi 10.1021/cc000051s
Jones, L., Schumm, J.S., and Tour, J.M., J. Org. Chem., 1997, vol. 62, no. 5, p. 1388. doi 10.1021/jo962336q
Moore, J.S., Acc. Chem. Res., 1997, vol. 30, no. 10, p. 402. doi 10.1021/ar950232g
Wirschun, W., Winkler, M., Lutz, K., and Jochims, J.C., J. Chem. Soc. Perkin Trans., 1998, vol. 2, no. 11, p. 1755. doi 10.1039/A801797B
Xie, X.H., Chen, J.Y., Xu, W.Q., He, E.X., and Zhan, S.Z., Inorg. Chim. Acta, 2011, vol. 373, no. 1, p. 276. doi 10.1016/j.ica.2011.02.091
Minaev, B., Baryshnikov, G., and Agren, H., Phys. Chem. Chem. Phys., 2014, vol. 16, no. 5, p. 1719. doi 10.1039/c3cp53806k
Evans, R.C., Douglas, P., Williams, J.A.G., and Rochester, D.L., J. Fluoresc., 2006, vol. 16, no. 2, p. 201. doi 10.1007/s10895-005-0037-9
Siu, P.K. M., Ma, D.L., and Che, C.M., Chem. Comm., 2005, no. 8, p. 1025. doi 10.1039/B414936J
Hush, N.S., J. Chem. Phys., 1958, vol. 28, no. 5, p. 962. doi 10.1063/1.1744305
Marcus, R.A., J. Chem. Phys., 1956, vol. 24, no. 5, p. 966. doi 10.1063/1.1742723
Li, X.N., Wu, Z.J., Si, Z.J., Zhang, H.J., Zhou, L., and Liu, X.J., Inorg. Chem., 2009, vol. 48, no. 16, p. 7740. doi 10.1021/ic900585p
Liu, Y., Gahungu, G., Sun, X., Su, J., Qu, X., and Wu, Z., Dalton Trans., 2012, vol. 41, no. 25, p. 7595. doi 10.1039/C2DT30342F
Malagoli, M. and Brédas, J.L., Chem. Phys. Lett., 2000, vol. 327, no. 1, p. 13. doi 10.1016/S0009-2614(00) 00757-0
Lin, B.C., Cheng, C.P., and Lao, Z.P.M., J. Phys. Chem.(A), 2003, vol. 107, no. 26, p. 5241. doi 10.1021/jp0304529
Hutchison, G.R., Ratner, M.A., and Marks, T.J., J. Am. Chem. Soc., 2005, vol. 127, no. 7, p. 2339. doi 10.1021/ja0461421
Liu, Y.Q., Sun, X.B., Gahungu, G., Qu, X.C., Wang, Y., and Wu, Z.J., J. Mater. Chem.(C), 2013, vol. 1, no. 23, p. 3700. doi 10.1039/C3TC30206G
Zhao, Y. and Truhlar, D.G., Theor. Chem. Acc., 2008, vol. 120, nos. 1–3, p. 215. doi 10.1007/s00214-007-0310-x
Becke, A.D., J. Chem. Phys., 1993, vol. 98, no. 7, p. 5648. doi 10.1063/1.464913
Lee, C., Yang, W., and Parr, R.G., Phys. Rev.(B), 1988, vol. 37, no. 2, p. 785. doi 10.1103/PhysRevB.37.785
Stephens, P.J., Devlin, F.J., Chabalowski, C.F., and Frich, M.J., J. Phys. Chem., 1994, vol. 98, no. 45, p. 11623. doi 10.1021/j100096a001
Adamo, C. and Barone, V., J. Chem. Phys., 1999, vol. 110, no. 13, p. 6158. doi 10.1063/1.478522
Ernzerhof, M. and Scuseria, G.E., J. Chem. Phys., 1999, vol. 110, no. 11, p. 5029. doi 10.1063/1.478401
Wang, L., Yu, X.H., Zhao, J.X., Zhang, Y.X., He, H.Q., and Zhang, J.L., Synthetic Met., 2013, vol. 175, p. 174. doi 10.1016/j.synthmet.2013.05.016
Wang, L., Zhang, Y.X., He, H.Q., and Zhang, J.L., Synthetic Met., 2013, vol. 167, p. 51. doi 10.1016/j.synthmet.2013.02.004
Hay, P.J. and Wadt, W.R., J. Chem. Phys., 1985, vol. 82, no. 1, p. 270. doi 10.1063/1.448799
Hay, P.J. and Wadt, W.R., J. Chem. Phys., 1985, vol. 82, no. 1, p. 299. doi 10.1063/1.448975
Hehre, W.J., Ditchfield, R., and Pople, J.A., J. Chem. Phys., 1972, vol. 56, no. 5, p. 2257. doi 10.1063/1.1677527
Liu, Y.Q., Sun, X.B., Si, Y.L., Qu, X.C., Wang, Y., and Wu, Z.J., RSC Adv., 2014, vol. 4, no. 12, p. 6284. doi 10.1039/C3RA46804F
Li, L.J., Liu, X.J., Feng, J., Song, S.Y., and Zhang, H.J., J. Mol. Model., 2014, 20, no. 10, p. 1. doi 10.1007/s00894-014-2437-8
Eddin, A.C., Planchat, A., Mennucci, B., Adamo, C., and Jacquemin, D., J. Chem. Theory Comput., 2013, vol. 9, no. 6, p. 2749. doi 10.1021/ct4000795
Andrae, D., Haeussermann, U., Dolg, M., Stoll, H., and Preuss, H., Theor. Chim. Acta, 1990, vol. 77, no. 2, p. 123. doi 10.1007/BF01114537
Miertus, Š., Scrocco, E., and Tomasi, J., Chem. Phys., 1981, vol. 55, no. 1, p. 117. doi 10.1016/0301-0104(81) 85090-2
Tomasi, J. and Persico, M., Chem. Rev., 1994, vol. 94, no. 7, p. 2027. doi 10.1021/cr00031a013
Verdolino, V., Cammi, R., Munk, B.H., and Schlegel, H.B., J. Phys. Chem. (B), 2008, vol. 112, no. 51, p. 16860. doi 10.1021/jp8068877
Scalmani, G., Barone, V., Kudin, K.N., Pomelli, C.S., Scuseria, G.E., and Frisch, M.J., Theor. Chem. Acc., 2004, vol. 111, nos. 2–6, p. 90. doi 10.1007/s00214-003- 0527-2
Kui, S.C.F., Chow, P.K., Cheng, G., Kwok, C.C., Kwong, C.L., Low, K.H., and Che, C.M., Chem. Commun., 2013, vol. 49, no. 15, p. 1497. doi 10.1039/C2CC37862K
Wu, Y., Shan, G.G., Li, H.B., Wu, S.X., Ren, X.Y., Geng, Y., and Su, Z.M., Phys. Chem. Chem. Phys., 2015, vol. 17, no. 4, p. 2438. doi 10.1039/C4CP04919E
Zhang, L., Tian, L., Li, M., He, R., and Shen, W., Dalton Trans., 2014, vol. 43, no. 17, p. 6500. doi 10.1039/C3DT53209G
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Wang, X., Wang, L., Li, J. et al. Theoretical study on electronic structures, spectra, and charge transporting properties of two Pt(II) complexes with triazenido ligands. Russ J Gen Chem 86, 2817–2826 (2016). https://doi.org/10.1134/S1070363216120458
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363216120458