Abstract
The reaction of 1-(o-methoxyphenyl)-3,4-diphenylcyclopentadienyl potassium with gadolinium chloride tetrahydrofuranate gives, depending on the stoichiometry, either tetranuclear complex [{[η5-(Ph2(o-CH3OC6H4)C5H2)Gd(Thf)]2(µ2-Cl)2(µ3-Cl)3K(Thf)}2] (I) or mononuclear complex [(Ph2(o-CH3OC6H4)C5H2)2GdCl] (II) (CIF files CCDC nos. 2116742 (I), 2116741 (II)). In complex I, the oxygen atom of the methoxy group is not coordinated to the gadolinium cation, whereas in complex II, the Gd3+ cation is coordinated to the oxygen atoms of both methoxy groups. Complex II crystallizes in the chiral space group P41212.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cyclopentadienyl lanthanide complexes play an important role in the organometallic chemistry of 4f elements and are the first known organic derivatives of lanthanides [1–4]. The importance of cyclopentadienyl complexes in lanthanide chemistry is due to the ease of modification of cyclopentadienyl ligand via replacement of the hydrogen atoms of the five-membered ring with various organic groups. Lanthanide complexes with unsubstituted and alkyl- and silyl-substituted cyclopentadienyl ligands have been most studied; arylcyclopentadienyl ligands still play a modest role in the lanthanide chemistry, despite their obvious benefits related to the diversity of possible modification pathways of these compounds by introduction of substituents into the aryl moiety [5, 6]. Previously, we showed that by using di-, tri-, and tetraphenylcyclopentadienyl ligands, it is possible to obtain various structural types of mono-, bis-, and tris-cyclopentadienyl complexes of gadolinium, neodymium, and terbium [7–9]. Owing to the presence of noncovalent interactions contacts involving prepurified phenyl substituents in the cyclopentadienyl ligands, various structural types of these complexes were formed, ranging from mononuclear and binuclear structures to coordination polymers [9].
The purpose of this study is to elucidate the coordination possibilities of polyaryl-substituted cyclopentadienyl ligands containing methoxyphenyl substituents. It was expected that methoxy groups capable of coordination to lanthanide ions would give rise to conceptually new complexes.
EXPERIMENTAL
All synthetic operations were carried out in the atmosphere of purified argon in anhydrous solvents using a SPEKS-GB2 glove box. Prior to use, THF was dried over NaOH and distilled from potassium/benzophenone. Hexane was distilled from potassium–sodium alloy/benzophenone. Toluene was distilled from sodium/benzophenone. GdCl3(THF)2.1 was prepared by a known procedure [10]. Benzyl potassium was obtained using a modification of the procedure reported in the literature [11]. 1-(o-Methoxyphenyl)-3,4-diphenylcyclopentadiene was obtained by a published procedure [12] and sublimed in a high vacuum. Elemental analysis was carried out on a Thermo Scientific FLASH 2000 CHNS/O Analyzer.
Synthesis of [{[η5-(Ph2(o-CH3OC6H4)C5H2)Gd(Thf)]2(µ2-Cl)2(µ3-Cl)3K(Thf)}2](Thf)3 (I). A solution of benzyl potassium (0.265 g, 2.04 mmol) in THF (10 mL) was slowly added with stirring to a solution of 1-(o-methoxyphenyl)-3,4-diphenylcyclopentadiene (0.648 g, 2 mmol) in THF (10 mL). The reaction mixture was stirred for 15 min, and the resulting solution of 1-(o-methoxyphenyl)-3,4-diphenylcyclopentadienyl potassium was slowly added to a stirred suspension of GdCl3(Thf)2.1 (0.830 g, 2 mmol) in THF (10 mL). The reaction mixture was stirred for 12 h and centrifuged. The solution was concentrated to a volume of 10 mL, and hexane (20 mL) was carefully added, avoiding mixing of the layers. The crystalline precipitate of I formed within 5 days was dried in a dynamic vacuum. The yield of I was 1.200 g (92%).
For C64H70O6Cl5Gd2 | ||
Anal. calcd., % | C, 52.77 | H, 4.97 |
Found, % | C, 52.69 | H, 4.63 |
The crystals suitable for X-ray diffraction were prepared by slow diffusion of hexane into a solution of I in tetrahydrofuran.
Synthesis of [(Ph2(o-CH3OC6H4)C5H2)2GdCl] (II). A solution of benzyl potassium (0.265 g, 2.04 mmol) in THF (10 mL) was slowly added with stirring to a solution of 1-(o-methoxyphenyl)-3,4-diphenylcyclopentadiene (0.648 g, 2 mmol) in THF (10 mL). The reaction mixture was stirred for 15 min, and the resulting solution of 1-(o-methoxyphenyl)-3,4-diphenylcyclopentadienyl potassium was slowly added to a stirred suspension of GdCl3(Thf)2.1 (0.415 g, 1 mmol) in THF (10 mL). The reaction mixture was stirred for 12 h and centrifuged. The solution was concentrated to dryness, and the resulting viscous oil was triturated with hexane. The precipitate was separated from the solution by centrifugation. Toluene (7 mL) was added to the precipitate, and the potassium chloride precipitate was separated by centrifugation. Hexane (30 mL) was carefully added to the solution, avoiding mixing of the layer. The crystalline precipitate of II formed within 7 days was dried in a dynamic vacuum. The yield of II was 0.582 g (69%).
For C48H38ClO2Gd | ||
Anal. calcd., % | C, 68.69 | H, 4.53 |
Found, % | C, 68.32 | H, 4.38 |
The crystals suitable for X-ray diffraction were prepared by slow diffusion of hexane into a solution of II in toluene.
X-ray diffraction analysis of complexes I and II was carried out on a Bruker Quest D8 diffractometer (MoKα radiation, graphite monochromator, ω-scan mode). The structures were solved by direct methods and refined by the least squares method in the full-matrix anisotropic approximation on \(F_{{hkl}}^{2}\). The absorption corrections were applied semiempirically on the basis of equivalent reflections. The disordered groups were refined using constraints on the atomic displacement parameters and positional parameters (DFIX and EADP). The hydrogen atoms in all structures were calculated and refined using the riding model. All calculations were carried out by the SHELXL-2014/2017 software package. The main crystallographic data and refinement parameters for compounds I and II are summarized in Table 1.
The atomic coordinates and other structural parameters were deposited with the Cambridge Crystallographic Data Centre (CCDC nos. 2116742 (I), 2116741 (II), deposit@ccdc.cam.ac.uk or http://www. ccdc.cam.ac.uk/data_request/cif).
RESULTS AND DISCUSSION
The reaction of a THF solution of the potassium salt of 1-(o-methoxyphenyl)-3,4-diphenylcyclopentadiene with a suspension of gadolinium trichloride tetrahydrofuranate gives, depending on the reactant ratio, either mono-cyclopentadienyl ate complex [{[η5-(Ph2(o-C6H4OCH3)C5H2)Gd(Thf)]2(µ2-Cl)2(µ3-Cl)3K(Thf)}2] (I) or bis-cyclopentadienyl complex [(Ph2(o-C6H4OCH3)C5H2)2GdCl] (II) (Scheme 1).

Scheme 1 .
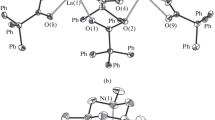
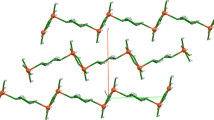
The structures of the products were established by X-ray diffraction. Tetranuclear complex I (Fig. 1, Table 2) consists of two {[η5-(Ph2(o-C6H4OCH3)C5H2)Gd(Thf)]2(µ2-Cl)2(µ3-Cl)3K(Thf)} moieties connected through two K−Cl bonds of the planar K2Cl2 unit. The Gd3+ cation (C.N. = 8) is η5‑coordinated to the cyclopentadienyl ligand, THF molecule, and four chloride ligands; one of the gadolinium cations in each moiety is surrounded by three µ2-chloride ligands and one µ3-chloride ligand, while the second cation is surrounded by two µ2-chloride ligands and two µ3-chloride ligands. Two carbon atoms of the phenyl ring of one of the two arylcyclopentadienyl ligands in each of the moieties form short contacts (C…K, 3.260(4)–3.396(3) Å) with the potassium cation. In complex I, the methoxy group of the phenyl substituent is not involved in intra- or intermolecular coordination to gadolinium, which is surprising, in view of the known oxophilicity of lanthanides [13]. As a result, the structure of I proves to be similar to the structure of the complex with the parent tri-phenylcyclopentadienyl ligand, [{[η5-(Ph3C5H2)-Gd(Thf)]2(µ2-Cl)2(µ3-Cl)3K(Thf)}2] (III) [9], devoid of electron-donating substituents. The methoxyphenyl group of the cyclopentadienyl ligand is arranged in space similarly to the phenyl group in the same position of the cyclopentadienyl ring in complex III.
It is of interest that, whereas the rotation angles of phenyl substituents in I and II are similar (30.1°−37.8° and 28.4°−39.3°), the rotation angle of the methoxyphenyl group is markedly greater (30.6°−32.8°) than that of the phenyl group in position 1 of complex III (18.1°−20.4°). This pronounced rotation of the methoxyphenyl substituent is surprising, since the planar arrangement is favorable not only for conjugation with the cyclopentadienyl ring, but also in terms of the possible intramolecular C−H…O contact with hydrogen of the cyclopentadienyl ligand (Cp). Analysis of the intramolecular contacts suggests that the oxygen atom is involved in the intramolecular contact with the bridging chloride ligand with Cl(1m)…O(1) and Cl(1m)…O(1A) distances of 3.170(2) and 3.220(2) Å, respectively (Fig. 2). This type of contacts is also indicated by the magnitude of the OClGd angles (161°−162°); this is in line with the possible charge transfer from the oxygen atom to the antibonding orbital of the Gd–Cl bond.
Complex II (Fig. 3), which belongs to bis-cyclopentadienyl derivatives, is built in a basically different way. The gadolinium cation in II is η5‑coordinated to two cyclopentadienyl ligands, a chloride anion, and oxygen atoms of both methoxy groups. In complex II, C.N.(Gd) = 9. The molecule of II crystallizes in the chiral space group P41212 and occupies a special position: a 2-fold axis that passes through the Gd−Cl bond. Crystallization in a chiral space group, in turn, implies the formation of a conglomerate: a mechanical mixture of enantiomers in which the chiral conformation is stabilized by Gd…O bonds. Like in the case of complex I, the rotation angles of the unsubstituted phenyl rings (25.2(2)° and 31.8(2)°) virtually do not differ from those in I or III (see above). The rotation angle of the methoxyphenyl substituent is, as expected, significantly larger (71.4(2)°) as a result of the Gd…O interaction. Although the Gd…O distance in II (2.617(2) Å) falls in the range of the longest Gd…O(Me)−Ph bond lengths (the average Gd…O distance is ~2.55 Å), the O−C bond length in II (1.391(5) Å) is much longer than that in I (1.362(3) Å).
Thus, in relation to the structure of complexes I and II, it was shown that the 1-(o-methoxyphenyl)-3,4-diphenylcyclopentadienyl ligand is coordinated to the central metal ion in conceptually different modes, depending on the type of the complex being formed (mono- or bis-cyclopentadienyl complex); in bis-cyclopentadienyl complex II, both methoxy groups are involved in coordination, which leads to increase in the gadolinium C.N. to 9 and to a regular lengthening of the Gd−CCp distances.
REFERENCES
Wilkinson, G. and Birmingham, J.M., J. Am. Chem. Soc., 1954, vol. 76, p. 6210.
Birmingham, J.M. and Wilkinson, G., J. Am. Chem. Soc., 1956, vol. 78, p. 42.
Maginn, R.E., Manastyrskyj, S., and Dubeck, M., J. Am. Chem. Soc., 1963, vol. 85, p. 672.
Manastyrskyj, S., Maginn, R.E., and Dubeck, M., Inorg. Chem., 1963, vol. 2, p. 904.
Yang, L., Ye, J., Xu, L., et al., RSC Adv., 2012, vol. 2, p. 11529.
Zhang, X., Ye, J., Xu, L., et al., J. Lumin., 2013, vol. 139, p. 28.
Minyaev, M.E., Vinogradov, A.A., Roitershtein, D.M., et al., J. Organomet. Chem., 2016, vol. 818, p. 128.
Roitershtein, D.M., Minyaev, M.E., Mikhaylyuk, A.A., et al., Russ. Chem. Bull., 2012, vol. 61, p. 1726.
Roitershtein, D.M., Puntus, L.N., Vinogradov, A.A., et al., Inorg. Chem., 2018, vol. 57, p. 10199.
Edelmann, F.T. and Poremba, P., Synthetic Methods of Organometallic and Inorganic Chemistry, Edelmann, F.T. and Herrmann, W.A., Eds., Stuttgart: Thieme, 1997, vol. 6, p. 34.
Lochmann, L. and Trekoval, J., J. Organomet. Chem., 1987, vol. 326, p. 1.
Hirsch, S.S. and Bailey, W.J.J., Org. Chem., 1978, vol. 43, p. 4090.
Bünzli, J.-C.G., Acc. Chem. Res., 2006, vol. 39, p. 53.
Funding
This study was supported by the Russian Science Foundation (grant no. 17-13-01357).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bardonov, D.A., Lysenko, K.A., Nifant’ev, I.E. et al. Synthesis and Structural Diversity of Gadolinium 1-(o-Methoxyphenyl)-3,4-diphenylcyclopentadienyl Complexes. Russ J Coord Chem 48, 295–300 (2022). https://doi.org/10.1134/S1070328422050013
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328422050013