Abstract
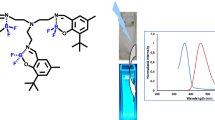
A novel Schiff base containing 1,8-naphthalimidyl group (L) and its boron complex C27H29BN4O2F2 (I) were successfully synthesized and their structures were confirmed by using elemental analysis, UV−Vis, ESI-MS, 1H NMR and 13C NMR spectroscopies. The fluorescence properties of new Schiff base L as well as its boron complex I were investigated. X-ray single crystal analysis of boron complex I (CIF file CCDC no. 1888435) reveals that the coordination of BF2 with one of the hydrazone group nitrogens (N(2)) along with the pyridyl nitrogen (N(3)) forms a six-membered ring. The boron atom adopts a tetrahedral geometry and the plane defined by F–B–F atoms is perpendicular to that of the central C(2)N(3) core. One-dimensional chains were formed along the b axis through weak π–π interactions and the adjacent molecular are stabilized by C–H⋅⋅⋅O hydrogen bonds interactions, forming a three-dimensional architecture. Luminescent properties reveal that the emission wavelength is blue-shifted whether in CH2Cl2 solution or in crystal state after the ligand was coordinated by BF2 fragment. The Stokes shift for I is about 73 nm, which may have been caused by the intramolecular hydrogen bonds dominated by the oxygen atom and the nitrogen atom and the distortion of the molecular structure.



Similar content being viewed by others
REFERENCES
Nelyubin, A.V., Klyukin, I.N., Zhdano, A.P., et al., Russ. J. Inorg. Chem., 2019, p. 1499. https://doi.org/10.1134/S003602361912012X
Avdeeva, V.V., Vologzhanin, A.V., Malinin, E.A., et al., Russ. J. Coord. Chem., 2019, vol. 45, p. 295. https://doi.org/10.1134/S1070328419040018
Sivaev, I.B., Russ. J. Inorg. Chem., 2019, vol. 64, p. 955. https://doi.org/10.1134/S003602361908014X
Burn, P.L., Lo, S.-C., and Samuel, I.D.W., Adv. Mater., 2007, vol. 19, p. 1675.
Saragi, T.P.I., Spehr, T., Siebert, A., et al., Chem. Rev., 2007, vol. 107, p. 1011.
Grimsdale, A.C., Chan, K.L., Martin, R.E., et al., Chem. Rev., 2009, vol. 109, p. 897.
Hatakeyama, T., Hashimoto, S., Seki, S., and Nakamura, M., J. Am. Chem. Soc., 2011, vol. 133, p. 18614.
van de Wouw, H.L., Lee, J.Y., Siegler, M.A., and Klausen, R.S., Org. Biomol. Chem., 2016, vol. 14, p. 3256.
Shimizu, M. and Hiyama, T., Chem. Asian J., 2010, vol. 5, p. 1516.
Hong, Y., Lam, J.W.Y., and Tang, B.Z., Chem. Commun., 2009, p. 4332.
Hong, Y., Lam, J.W.Y., and Tang, B.Z., Chem. Soc. Rev., 2011, vol. 40, p. 5361.
Zhang, D., Wen, Y., Xiao, Y., et al., Chem. Commun., 2008, p. 4777.
Yang, Y., Hughes, R.P., and Aprahamian, I., J. Am. Chem. Soc., 2012, vol. 134, p. 15221.
Zhang, Z., Chen, C., Chen, Y., et al., Angew. Chem. Int. Ed., 2018, vol. 57, p. 9880.
Liu, H., Lu, H., Zhou, Z., et al., Chem. Commun., 2015, vol. 51, p. 1713.
Zhu, W.J., Qin, Z.J., Bai, Y., and Dang, D.B., Russ. J. Coord. Chem., 2018, vol. 44, p. 425. https://doi.org/10.1134/S1070328418070096
Sergienko, V.S., Koksharova, T.V., Surazhskaya, M.D., and Skakun, T.S., Russ. J. Inorg. Chem., 2018, vol. 63, p. 1171. https://doi.org/10.1134/S0036023618090176
Satta, Y., Nishiyabu, R., James, T.D., and Kubo, Y., Tetrahedron, 2017, vol. 73, p. 2053.
Gudeika, D., Lygaitis, R., Mimait, V., et al., Dyes Pigments, 2011, vol. 91, p. 13.
SAINT, Version 6.45, Bruker Analytical X-ray Systems Inc., 2003.
Sheldrick, G.M., SHELXS-97, Program for Crystal Structure Solution and Refinement, Göttingen: Univ. of Göttingen, 1997.
Sheldrick, G.M., SHELXL, Crystal Structure Refinement Program, Göttingen: Univ. of Göttingen, 2013.
Satta, Y., Nishiyabu, R., James, T.D., and Kubo, Y., Tetrahedron, 2017, vol. 73, p. 2053.
Funding
We appreciate the financial support from Shandong Provincial Natural Science Foundation (ZR2018LB015); the Scientific Research Foundation of Qingdao Agricultural University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gao, L.B. Synthesis, Structure and Photophysical Properties of 1,8-Naphthalimidyl-Derived Schiff Base and Its Boron Complex. Russ J Coord Chem 47, 88–94 (2021). https://doi.org/10.1134/S1070328420120039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328420120039