Abstract
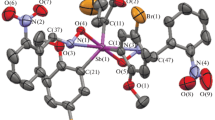
Antimony aroxides [Ph3SbOC6H3(NO2)2-2,5]2O (I) and [(4-MeC6H4)3SbOC6H3(NO2)2-2,5]2O (II) were synthesized by the oxidation of triarylantimony with tert-butyl hydroperoxide in the presence of 2,5-dinitrophenol. The SbOSb fragments in compounds I and II are bent (the corresponding angles are 139.70(10)° and 142.32(12)°). The Sb−Оbr bonds (1.973(3), 1.980(3) Å in I; 1.975(2), 1.977(2) Å in II) are substantially shorter than Sb−Ot (2.211(3), 2.213(3) and 2.191(2), 2.191(2) Å in I and II, respectively). Aroxides Ar4SbOC6H3(NO2)2-2,5 (Ar = Ph (III) and 4-MeC6H4 (IV)) and carbonates (Ar4Sb)2CO3 (Ar = Ph (V) and 4-MeC6H4 (VI)) are formed from Ar5Sb and compounds I and II in the presence of oxygen and carbon dioxide, respectively. In the trigonal bipyramidal molecules of compounds III and IV, the СSbO axial angles are 175.80(7)° and 176.86(10)° (Sb−O 2.290(2) and 2.342(2) Å, respectively). One of the antimony atoms in compound V and in crystallographically independent molecules of two types of compound VI is pentacoordinated (the Sb−Oax bond is 2.245(6) Å in V and is 2.263(3) and 2.263(3) Å in VIа and VIb, respectively), whereas the second antimony atom is hexacoordinated (Sb−O 2.249(6) and 2.273(5) Å in V; 2.216(2), 2.251(2) Å and 2.217(2), 2.251(2) Å in VIа and VIb). The crystallographic data are deposited with the Cambridge Crystallographic Data Centre (СIF files ССDС nos. 1890704 (I), 1890706 (II), 1890713 (III), 1890714 (IV), 994519 (V), and 994177 (VI)).



Similar content being viewed by others
REFERENCES
Hadjikakou, S.K., Ozturk, I.I., Banti, C.N., et al., J. Inorg. Biochem., 2015, vol. 153, p. 293.https://doi.org/10.1016/j.jinorgbio.2015.06.006
Ali, M.I., Rauf, M.K., Badshah, A., et al., Dalton Trans., 2013, vol. 42, p. 16733.https://doi.org/10.1039/C3DT51382C
Zhang, X.-Y., Cui, L., Zhang, X., et al., J. Mol. Struct., 2017, vol. 1134, p. 742.https://doi.org/10.1016/j.molstruc.2017.01.039
Gushchin, A.V., Grunova, E.V., Moiseev, D.V., et al., Izv. Akad. Nauk, Ser. Khim., 2003, no. 6, p. 1302.
Sharutin, V.V., Sharutina, O.K., Efremov, A.N., et al., Russ. J. Inorg. Chem., 2018, vol. 63, no. 2, p. 174. https://doi.org/10.1134/S0036023618020195
Sharutin, V.V., Sharutina, O.K., and Efremov, A.N., Russ. J. Inorg. Chem., 2018, vol. 63, no. 3, p. 343.https://doi.org/10.1134/S0036023618030208
Sharutin, V.V., Sharutina, O.K., Senchurin, V.S., et al., Vestnik YuUr.Gos. Univ. Ser. Khim., 2014, vol. 6, no. 4, p. 14.
SMART and SAINT-Plus. Version 5.0. Data Collection and Processing Software for the SMART System, Madison: Bruker AXSInc., 1998.
SHELXTL/PC. Version 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data, Bruker AXS Inc., Madison, 1998.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, p. 339.https://doi.org/10.1107/S0021889808042726
Li, N., Qiu, R., Zhang, X., et al., Tetrahedron, 2015, vol. 71, p. 4275.https://doi.org/10.1016/j.tet.2015.05.013
Gibbons, M.N. and Sowerby, D.B., J. Organomet. Chem., 1998, vol. 555, p. 271.https://doi.org/10.1016/S0022-328X(97)00759-6
Ruther, R., Huber, F., and Preut, H., Angew. Chem., Int. Ed. Engl., 1987, vol. 26, p. 906.https://doi.org/10.1002/anie.198709061
Preut, H., Ruther, R. and Huber, F., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1986, vol. 42, p. 1154.https://doi.org/10.1107/S010827018609306X
Gibbons, M.N. and Sowerby, D.B., J. Chem. Soc., Dalton Trans. (1972–1999), 1997, p. 2785.
Effendy Grigsby, W.J., Hart, R.D., et al., Aust. J. Chem., 1997, vol. 50, p. 675.https://doi.org/10.1071/C96042
Quan, L., Yin, H., and Wang, D., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, vol. 65.https://doi.org/10.1107/S1600536808042335
Quan, L., Yin, H., and Wang, D., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, vol. 64.https://doi.org/10.1107/S1600536808000676
Ebina, F., Ouchi, A., Yoshino, Y., et al., Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1978, vol. 34, p. 2134.https://doi.org/10.1107/S0567740878007578
Mahon, M.F., Molloy, K.C., Omotowa, B.A., et al., J. Organomet.Chem., 1998, vol. 560, p. 95.https://doi.org/10.1016/S0022-328X(98)00488-4
Abakumov, G.A., Vavilina, N.N., Kursky, Yu.A., et al., Russ. Chem. Bull., 2007, vol. 9, p. 1813.
Perpetuo, G.J. and Janczak, J., Acta Crystallogr., Sect. C: Crystal Struct. Commun., 2005, vol. 61, p. o165.https://doi.org/10.1107/S0108270105001253
Sharutin, V.V., Sharutina, O.K., Senchurin, V.S., et al., Russ. J. Coord. Chem., 2001, vol. 27, no. 9, p. 669.https://doi.org/10.1023/A:1017909824029
Batsanov, S.S., Russ. J. Inorg. Chem., 1991, vol. 36, no. 12, p. 3015.
Ferguson, G. and Hawley, D.M., Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, vol. 30, no. 1, p. 103.https://doi.org/10.1107/S0567740874002299
Sharutin, V.V., Sharutina, O.K., Platonova, T.P., et al., Russ. J. Gen. Chem., 2001, vol. 71, no. 10, p. 1550.https://doi.org/10.1023/A:1013938600798
Funding
South Ural State University thanks the Ministry of Science and Higher Education of the Russian Federation for financial support, project no. 4.6151.2017/8.9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K. & Efremov, A.N. µ2-Oxo-Bis[(2,5-Dinitrophenoxo)triarylantimony]: Syntheses, Structures, and Reactions with Pentaarylantimony. Russ J Coord Chem 46, 42–52 (2020). https://doi.org/10.1134/S1070328419120066
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419120066