Abstract
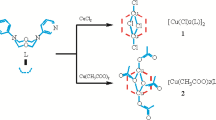
Complexes [CuL1Cl2] (I), [CuL2Cl2] · EtOH (II), and Cu2L3Cl4 (III) containing esters of the N-derivatives of optically active amino acids based on (+)-3-carene (L1, L2) and (‒)-α-pinene (L3) are synthesized. The crystal and molecular structures of compounds I and II are determined by X-ray diffraction analyses (CIF files CCDC nos. 1560071 (I), 1560072 (II)). The crystal structure of compound I consists of mononuclear complex molecules. In the structure of compound II, the unit cell contains two crystallographically independent molecules of mononuclear complex [CuL2Cl2] and two EtOH molecules. Ligands L1 and L2 perform the tridentate-chelating function by the N atoms of the NH and NOH groups and by the O atom of the C=O group. In compounds I and II, the coordination polyhedra Cl2N2O of the Cu atoms are trigonal bipyramid. According to the data of IR and electronic spectroscopy, binuclear complex III has similar coordination polyhedra. The experimental values of μeff for compounds I, II, and III at 300 K are 1.93, 1.88, and 2.71 μB. For complex III, the μeff(T) dependence in a range of 2–300 K indicates a weak ferromagnetic exchange interaction.
Similar content being viewed by others
References
Williams D.R., The Metals of Life: The Solution Chemistry of Metal Ions in Biological Systems, London: Van Nostrand-Reinhold, 1971.
Nozdryukhina, L.R. Biologicheskaya rol’ mikroelementov v organizme zhivotnykh i cheloveka (Biological Role of Trace Minerals in the Animal and Human Body), Moscow: Nauka, 1977.
Hughes, M.N., Inorganic Chemistry of Biological Processes, Chichester: Wiley, 1981.
Armaroli, N., Accorsi, G., Cardinali, F., and Listorty, A., Top. Curr. Chem., 2007, vol. 280, p.69.
Dabrowiak, C.D., Metals in Medicine, New York: Wiley, 2009.
Marzano, C., Pellei, M., Tisato, F., and Santini, C., Anti-Cancer Agents Med. Chem., 2009, vol. 9, no. 2, p.185.
Santini, C., Pellei, M., Gandin, V., et al., Chem. Rev., 2014, vol. 114, p.815.
Blower, P.J., Annu. Rep. Progr. Chem. A, 2003, vol. 99, p.589.
Krajc'ova, D., Melnik, M., Havránec, E., et al., J. Coord. Chem., 2014, vol. 67, no. 9, p. 1493.
Donnelly, P.S., Dalton Trans., 2011, vol. 40, no. 5, p.999.
Garbutcheon-Singh, K.B., Grant, M.P., Harper, B.W., et al., Curr. Top. Med. Chem., 2011, vol. 11, p.521.
Allardyce, C.S. and Dyson, P.J., Dalton Trans., 2016, vol. 45, no. 8, p. 3201.
Sharma, S., Chauhan, M., Jamsheera, A., et al., Inorg. Chim. Acta, 2017, vol. 458, p.8.
Zelewsky, A. and Mamula, O., Dalton Trans., 2000, no. 8, p.219.
Mamula, O. and von Zelewsky, A., Coord. Chem. Rev., 2003, vol. 242, p.87.
Larionov S.V. and Tkachev A.V., Ros. Khim. Zh., 2004, vol. 48, no. 4, p.154.
Larionov S.V., Russ. J. Coord. Chem., 2012, vol. 38, no. 1, p.1.
Fernandes, T.A., Mendes, F., Roseiro, A.P.S., et al., Polyhedron, 2015, vol. 87, p.215.
Kokina, T.E., Agafontsev, A.M., Marenin, K.S., et al., Koord. Khim., 2015, vol. 41, no. 10, p.604.
Kokina, T.E., Glinskaya, L.A., Marenin, K.S., et al., Koord. Khim., 2017, vol. 43, no. 4, p.212.
Marenin, K.S., Gatilov, Yu.V., Agafontsev, A.M., Tkachev, A.V., Steroids, 2017, vol. 117, p.112.
Sheldrick, G.M., SHELX-97 Programs for Crystal Structure Analysis (Release 97–2), Göttingen: Univ. of Göttingen, 1997.
Petukhov, P.A., Bagryanskaya, I.Yu., Gatilov, Yu.V., and Tkachev, A.V., Mend. Commun., 2000, vol. 10, no. 6, p.209.
Kokina, T.E., Klevtsova, R.F., Glinskaya L.A., et al., Izv. Akad. Nauk, Ser. Khim., 2010, no. 4, p.698.
Batsanov, S.S., Zh. Neorg. Khim., 1991, vol. 36, no. 12, p. 3015.
Lever, A.B.P., Inorganic Electronic Spectroscopy, Amsterdam: Elsevier, 1984.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.A. Bryleva, L.A. Glinskaya, K.S. Marenin, A.S. Bogomyakov, D.A. Piryazev, A.V. Tkachev, S.V. Larionov, 2018, published in Koordinatsionnaya Khimiya, 2018, Vol. 44, No. 1, pp. 28–37.
Rights and permissions
About this article
Cite this article
Bryleva, Y.A., Glinskaya, L.A., Marenin, K.S. et al. Copper(II) Complexes with Chiral Ligands Containing Fragments of Monoterpenoids and Amino Acid Esters. Russ J Coord Chem 44, 117–126 (2018). https://doi.org/10.1134/S1070328418020033
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328418020033