Abstract
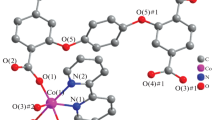
The reaction of different macrocyclic metallic tectons and dicarboxylic acid ligand yielded six new coordination polymers, namely, {[(NiL1)(4,4'-Bpdc)] • DMF • 2.5H2O} n (I), {[(NiL2)(4,4'-Bpdc)] • DMF • 2.5H2O} n (II), [(NiL3)2(4,4'-Bpdc)1.5][(NiL3)(4,4'-Bpdc)] • ClO4 • 28H2O (III), {[(NiL4)(4,4'-Bpdc)] • 4H2O} n (IV), {[(NiL5)(4,4'-Tpdc)] • 5H2O} n (V), {[(NiL3)(4,4'-Tpdc)]} n (VI) (L1 = 1,4,7,9,12,14-hexaaza-tricyclo[12.2.1.14.7]octadecane, L2 = 1,3,10,12,15,18-hexaazatetracyclo[16.2.1.112.15.04.9]docosane, L3 = 11-methyl-1,4,8,10,13,15-hexaaza-tricyclo[13.3.1.14.8]icosane, L4 = 1,3,10,12,16,19-hexaazate-tracyclo[17.3.1.1.12.16,04.9]tetracosane, L5 = 1,4,8,10,13,15-hexaaza-tricyclo[13.3.1.14.8]icosane, 4,4'-Bpdc = 4,4'-biphenyldicarboxylic acid and 4,4'-Tpdc = 4,4'-terphenyldicarboxylic acid) (CIF files CCDC nos. 1055545–1055550 for I–VI, respectively). Except for the different conformations of the macrocyclic metallic tectons or dicarboxylic acid ligands, complexes I–VI crystallized under the same environment, however, they exhibit diverse packing mode of infinite 1D coordination polymers, showing macrocyle or dicarboxylic acid ligand regulated self-assemble. The solid states UV-Vis for complexes I–VI also have been investigated.
Similar content being viewed by others
References
Choi, H.J., Lee, T.S., and Suh, M.P., Angew. Chem. Int. Ed., 1999, vol. 38, p. 1405.
Moon, H.R., Kim, J.H., and Suh, M.P., Angew. Chem. Int. Ed., 2005, vol. 44, p. 1261.
Clérac, R., Miyasaka, H., Yamashita, M., and Coulon, C., J. Am. Chem. Soc., 2002, vol. 124, p. 12837.
Liu, T.F., Fu, D., Gao, S., et al., J. Am. Chem. Soc., 2003, vol. 125, p. 13976.
Bogani, L., Sangregorio, C., Sessoli, R., and Gatteschi, D., Angew. Chem., Int. Ed., 2005, vol. 44, p. 5817.
Zheng, Y.Z., Tong, M.L., Zhang, W.X., and Chen, X.M., Angew. Chem., Int. Ed., 2006, vol. 45, p. 6310.
Jiang, X., Tao, B., Yu, X.L., et al., RSC Advances, 2015, vol. 5, p. 19034.
Noro, S., Daisuke, T., Hirotoshi, S., et al., Chem. Mater., 2009, vol. 14, p. 3346.
Cui, Y., Lee, S.J., and Lin, W.B., J. Am. Chem. Soc., 2003, vol. 125, p. 6014.
Yuan, G.Z., Zhu, C.F., Liu, Y., et al., J. Am. Chem. Soc., 2009, vol. 131, p. 10452.
Yan, G., Zhou, G.X., Yu, X.L., et al., J. Mol. Struct., 2014, vol. 1074, p. 393.
Tao, B., Jiang, X., Xia, H., and Cheng, H.F., J. Mol. Struct., 2012, vol. 1011, p. 15.
Yang, Q.Y., Zheng, S.R., Yang, R., et al., CrystEng-Comm., 2009, vol. 11, p. 680.
Carlucci, L., Ciani, G., and Proserpio, D.M., Coord. Chem. Rev., 2003, vol. 246, p. 247.
Jiang, X., Tao, B., Xia, H., and Liao, G.Y., CrystEng-Comm., 2012, vol. 14, p. 3271.
Zhang, B., Ni, Z.H., Cui, A.L., and Kou, H.Z., New. J. Chem., 2006, vol. 30, p. 1327.
Kou, H.Z., Gao, S., Ma, B.Q., and Liao, D.Z., Chem. Commun., 2000, p. 1309.
Suh, M.P., Kang, S.G., Gocdken, V.L., and Park, S., Inorg. Chem., 1991, vol. 30, p. 365.
Choi, K.Y., Chun, K.M., Lee, K.C., and Kim, J., Polyhedron, 2002, vol. 21, p. 1913.
Sujatha, S., Balasubramanian, S., and Varghese, B., Polyhedron, 2009, vol. 28, p. 3723.
Choi, K.Y., J. Coord. Chem., 2003, vol. 56, p. 481.
Lee, D.W., Suh, M.P., and Lee, J.W., J. Chem. Soc., Dalton. Trans., 1997, p. 577.
SMART and SADABS, Madison (WI): Bruker AXS Inc., 1998.
Sheldrick, G.M., SHELXL-97, Program for the Solution of Crystal Structure, Göttingen: Univ. of Göttingen, 1997.
Sheldrick, G.M., SHELXS-97, Program for the Refinement of Crystal Structure, Göttingen (Germany): Univ. of Göttingen, 1997.
Spek, A.L., PLATON, A Multipurpose Crystallographic Tool, The Netherlands: Utrecht Univ., 2006.
Ma, B.Q., Sun, H.L., and Gao, S., Chem. Commun., 2004, p. 2220.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Zhou, G.X., Wang, X.Y., Yan, G. et al. Syntheses, crystal structures, and properties of various one- dimensional coordination polymers based on macrocyclic metallic tectons and dicarboxylic acid ligand. Russ J Coord Chem 42, 461–469 (2016). https://doi.org/10.1134/S1070328416060105
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328416060105