Abstract
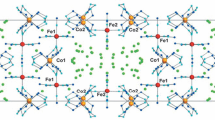
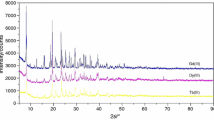
Binary complexes [Co(A)6][M(C2O4)3] (A = NH3, 1/2C2H8N2, M = Fe, Cr) are synthesized, and their physicochemical properties and thermal decomposition in air, argon, and hydrogen are studied. The qualitative and quantitative analyses are carried out for the solid and gaseous thermolysis products. Similar regularities are revealed in the behavior of complexes [Co(NH3)6][Fe(C2O4)3] (I), [Co(En)3][Fe(C2O4)3] (II), [Co(NH3)6][Cr(C2O4)3] (III), and [Co(En)3][Cr(C2O4)3(IV). The solid thermolysis product for complexes I and III in argon at 225 and 300°C is Co(NH2)2M(C2O4)2, respectively; and that for complexes II and IV at 280 and 380°C is Co(En)2M(C2O4)2. The gaseous thermolysis products are CO and CO2, NH3, En partially isolated upon thermal destruction, other products of En destruction, and En itself. Complex III forms the most highly dispersed solid products in a range of 300–400°C.
Similar content being viewed by others
References
Pechenyuk, S.I., Domonov, D.P., Rogachev, D.L., and Belyaevskii, A.T., Russ. J. Inorg. Chem., 2007, vol. 52, no. 7, p. 1033.
Pechenyuk, S.I., Gosteva, A.N., Domonov, D.P., and Makarova, T.I., Vestn. Yuzhno-Ural’Skogo Gos. Un-ta, Ser. Khim., 2012, no. 24, p. 4.
Pechenyuk, S.I., Domonov, D.P., Gosteva, A.N., et al., Russ. J. Coord. Chem., 2012, vol. 38, no. 9, p. 596.
Pechenyuk, S.I., Domonov, D.P., Gosteva, A.N., et al., Izv. Sankt-Ptetrburgsk. Gos. Tekh. Int. (TU), 2012, vol. 15(41), p. 18.
Pechenyuk, S.I. and Gosteva, A.N., Russ. J. Coord. Chem., 2014, vol. 40, no. 8, p. 547.
Pechenyuk, S.I., Domonov, D.P., and Belyaevskii, A.T., Russ. J. Inorg. Chem., 2008, vol. 53, no. 8, p. 1221.
Pechenyuk, S.I., Domonov, D.P., Avedisyan, A.A., and Ikorskii, S.V., Russ. J. Inorg. Chem., 2010, vol. 55, p. 734.
Domonov, D.P., Cand. Sci. Dissertation (Chemistry), Novosibirsk: Inst. Inorg. Chem. Sib. Branch of the RAS, 2009.
Matyukha, V.A. and Zhiganov, A.N., Oksalaty perekhodnykh metallov (Transition Metal Oxalates), Moscow: IzdAT, 2012.
Handbuch der Pröparativen Anorganische Chemie, Brauer, G., Ed., vol. 3, Stuttgart: F. Enke, 1962.
K. Nakamoto, Infrared Spectra and Raman Spectra of Inorganic and Coordination Compounds, New York: Wiley, 1986.
Fritz, J.S. and Schenk, G.H., Quantitative Analytical Chemistry, Boston: Allyn and Bacon, 1974.
JCPDS-ICDD Card, Newtown Square (PA, USA): International Centre for Diffraction Data, 2002.
Kohata, S., Asakawa, M., Maeda, T., et al., Analyt. Sci., 1986, vol. 2, p. 325.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.I. Pechenyuk, Yu.P. Semushina, N.L. Mikhailova, Yu.V. Ivanov, 2015, published in Koordinatsionnaya Khimiya, 2015, Vol. 41, No. 3, pp. 157–162.
Rights and permissions
About this article
Cite this article
Pechenyuk, S.I., Semushina, Y.P., Mikhailova, N.L. et al. Binary complexes [Co(A)6][M(C2O4)3] (A = NH3, 1/2C2H8N2, M = Fe, Cr): Synthesis, properties, and thermal decomposition. Russ J Coord Chem 41, 175–181 (2015). https://doi.org/10.1134/S1070328415020086
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328415020086