Abstract
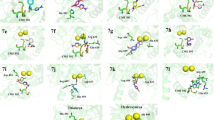
A new series of 5,6-dichloro-benzimidazoles containing thiophene ring derivatives of thiosemicarbazides and triazoles were designed, synthesized and characterized by spectral methods like as IR, 1H-NMR, 13C-NMR and elemental analysis. The compounds antiurease activity studies have done according to VanSlyke method and IC values were calculated in μM unit. All newly synthesized compounds containing thiophene ring showed urease inhibitory activity with IC50 values between 1.52 and 0.07 µM. 5-{[5,6-Dichloro-1-(2-thienylmethyl)-1H-benzimidazol-2-yl]methyl}-4-methyl-4H-1,2,4-triazol-3-thiol (Va) proved to be the most potent enzyme inhibition activity with IC50 = 0.07 ± 0.008 μM. Especially compounds (Va–f) have best results with triazole ring. All synthesized compounds were docked at the active sites of the Jack bean urease enzyme to investigate the reason of the inhibitory activity and the possible binding interactions of enzyme-ligand complexes. 2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-(4-clorophenyl)hydrazine carbothioamide (IVe), which has the second highest in vitro urease inhibitory activity with an IC50 of 0.11 μM compared to other compounds, has the highest binding energy of –8.97 kcal/mol.

Similar content being viewed by others
REFERENCES
Palit, R., Kumar, R., Saraswat, N., Wal, A., and Upadhyaya, P.K., Res. Ayurveda Phar., 2017, vol. 7, pp. 68–73.
Mabkhot, N.Y., Showiman, S.S., Soliman, G.H.A., and Aldamen, M.A., Chem. Cent. J., 2017, vol. 11. https://doi.org/10.1186/s13065-017-0280-6
Yılmaz, F., Menteşe, E., and Baltaş, N., Lett. Drug. Dis. Dis., 2017, vol. 14, pp. 201–208. https://doi.org/10.2174/1570180813666160609082633
Menteşe, E., Emirik, M., and Sökmen, B.B., Bioorg. Chem., 2019, vol. 86, pp. 151–158. https://doi.org/10.1016/j.bioorg.2019.01.061
Ragavendran, J., Sriram, D., Patil, S., Reddy, I.V., Bharathwajan, N., Stables, J., and Yogeeswari, P., Eur. J. Med. Chem., 2007, vol. 42, pp. 146–151. https://doi.org/10.1016/j.ejmech.2006.08.010
Polivka, Z., Holubek, J., Svatek, E., Metys, J., and Protiva, M., Collect. Czech. Chem. Commun., 1984, vol. 49, pp. 122–123. https://doi.org/10.1002/CHIN.198427237
Yogeeswari, P., Thirumurugan, R., Kavya, R., Samuel, J.S., Stables, J., and Siram, D., Eur. J. Med. Chem., 2004, vol. 39, pp. 729–734. https://doi.org/10.1016/j.ejmech.2004.03.008
Paramashivappa, R., Phani Kumar, P., Subba Rao, P.V., and Srinivasa Rao, A., Bioorg. Med. Chem. Lett., 2003, vol. 13, pp. 657–660. https://doi.org/10.1016/s0960-894x(02)01006-5
Keter, F. and Darkwa, J., Biometals, 2012, vol. 25, pp. 9–21. https://doi.org/10.1007/s10534-011-9496-4
Mobley, H.L.T., Island, M.D., and Hausinger, R.P., Microbiol. Rev., 1995, vol. 59, pp. 451–480. https://doi.org/10.1128/mr.59.3.451-480.1995
Maroney, M.J. and Ciurli, S., Chem. Rev., 2014, vol. 114, pp. 4206–4228. https://doi.org/10.1021/cr4004488
Mobley, H. and Hausinger, R., Microbiol. Mol. Biol. Rev., 1989, vol. 53, pp. 85–108.
Karplus, P.A., Pearson, M.A., and Hausinger, R.P., Acc. Chem. Res., 1997, vol. 30, pp. 330–337. https://doi.org/10.1021/ar960022j
Lodhi, M.A., Ajmal, M., Choudhary, M.I., Ahmed, V.U., Nat. Prod. Res., 2007, vol. 21, pp. 721–725. https://doi.org/10.1080/14786410600906913
Velík, J., Baliharová, V., Fink-Gremmels, J., Bull, S., Lamka, J.L., and Skálová, Res. Vet. Sci., 2004, vol. 76, pp. 95–108. https://doi.org/10.1016/j.rvsc.2003.08.005
Amtul, Z., Rahman, A., Siddiqui, R., and Choudhary, M.I., Curr. Med. Chem., 2002, vol. 9, pp. 1323–1348. https://doi.org/10.2174/0929867023369853
Saeed, A., Imran, A., Channar, P.A., Shahid, M., Mahmood, W., and Iqbal, J., Chem. Biol. Drug. Des., 2015, vol. 85, pp. 225–230. https://doi.org/10.1111/cbdd.12379
Akyüz, G., Cumhuriyet Sci. J., 2021, vol. 42, pp. 649–651. https://doi.org/10.17776/csj.867783
Shah, R. and Verma, P.K., Chem. Cent. J., 2018, vol. 12, p. 137. https://doi.org/10.1186/s13065-018-0511-5
Noreen, M., Rasool, N., Gull, Y., Zubair, M., Mahmood, T., Ayub, K., Nasim, F.U., Yaqoob, A., Zia-Ul-Haq, M., and de Feo, V., Molecules, 2015, vol. 20, pp. 19914–19928. https://doi.org/10.3390/molecules201119661
Khan, K.M., Wadood, A., Ali, M., Ul-Haq, Z., Lodhi, M.A., Khan, M., Perveen, S., and Choudhary, I., J. Mol. Graph. Mod., 2010, vol. 28, pp. 792–798. https://doi.org/10.1016/j.jmgm.2010.02.004
Khan, I., Ali, S., Hameed, S., Rama, N.H., Hussain, M.T., and Wadood, A., Eur. J. Med. Chem., 2010, vol. 45, pp. 5200–5207. https://doi.org/10.1016/j.ejmech.2010.08.034
Özil, M., Bodur, O., Ülker, S., and Kahveci, B., Chem. Heterocycl. Comp., 2015, vol. 51, pp. 88–96. https://doi.org/10.1007/s10593-015-1664-y
Akhtar, T., Hameed, S., Khan, K.M., Khan, A.L., and Choudhary, M., J. Enzym. Inh. Med. Chem., 2010, vol. 25, p. 572. https://doi.org/10.3109/14756360903389864
Menteşe, E., Emirik, M., and Sökmen, B.B., Bioorg. Chem., 2019, vol. 86, pp. 151–158.
Arshad, T., Khan, K.M., Rasool, N., Salar, U., Hussain, S., Asghar, H., Ashraf, M., Wadood, A., Riaz, M., Perveen, S., Taha, M., and Ismail, N.H., Bioorg. Chem., 2017, vol. 72, pp. 21–31. https://doi.org/10.1016/j.bioorg.2017.03.007
Menteşe, E., Bektaş, H., Emirik, M., Sökmen, B.B., and Kahveci, B., Bioorg. Med. Chem. Lett., 2017, vol. 27, pp. 3014–3018. https://doi.org/10.1016/j.bmcl.2017.05.019
Schrödinger, Suite 2009 Induced Fit Docking Protocol; Glide version 5.5, S., LLC, New York, NY, 2009; Prime version 2.1, Schrödinger, LLC, New York, NY, 2009.
Balasubramanian, A. and Ponnuraj, K., J. Mol. Biol., 2010, vol. 400, pp. 274–283. https://doi.org/10.1016/j.jmb.2010.05.009
Akyüz, G., Menteşe, E., Emirik, M., and Baltaş, N., Bioorg. Chem., 2018, vol. 80, pp. 121–128.
Van Slyke, D.D. and Archibald, R.M., J. Biol. Chem., 1944, vol. 154, pp. 623–628.
Funding
This work was supported by Recep Tayyip Erdogan University Scientific Research Project Unit (BAP) under the project number of FBA-2020-1128.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Akyüz, G., Emirik, M., Sökmen, B.B. et al. Synthesis and Docking Studies of Novel Benzimidazole Derivatives Containing Thiophene and Triazole Rings as Potential Urease Inhibitors. Russ J Bioorg Chem 48 (Suppl 1), S87–S95 (2022). https://doi.org/10.1134/S106816202301003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202301003X