Abstract
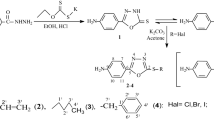
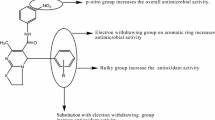
A series of Schiff bases of indole-3-carbaldehyde were synthesized by the condensation reaction of indole-3-carbaldehyde with different aryl amines. The reaction of indole-3-carbaldehyde with different aryl amines was carried out in the presence of glacial acetic acid. All the synthesized compounds were characterized with microanalytical data 1H and 13C NMR, FT-IR spectroscopy and Mass spectrometry and screened for their in vitro microbial activity against bacterial strain Dickeya species as well as fungal culture Fusarium oxysporum using agar well diffusion method. The standard used for bacterial and fungal cultures were Carbendazim and Gentamicin, respectively. The results revealed that the synthesized Schiff base N‑((indol-1H-3-yl)methylene)-4-nitrobenzenamine exhibited the maximum activity against both bacteria (MIC = 2000 µg/mL) as well as fungus (MFC = 5000 µg/mL) cultures as compared with all other synthesized compounds.





Similar content being viewed by others
REFERENCES
Jain, N., Utreja, D., and Dhillon, N.K., Russ. J. Org. Chem., 2019, vol. 55, pp. 845–851. https://doi.org/10.1134/S1070428019060150
Nguyen, L.H.T., Nguyen, T.T.T., Dang, M.H.D., Tran, P.H., and Doan, T.L.H., Mol. Catal., 2021, vol. 499, p. 111291. https://doi.org/10.1016/j.mcat.2020.111291
Anamika, Utreja, D., Ekta, Jain, N., and Sharma, S., Curr. Org. Chem., 2018, vol. 22, pp. 2507–2534. https://doi.org/10.2174/1385272822666181029102140
Utreja, D., Kaur, J., Kaur, K., and Jain, P., Mini-Rev. Org. Chem., 2020, vol. 17, pp. 991–1041. https://doi.org/10.2174/1570193X17666200129094032
Sravanthi, T.V. and Manju, S.L., J. Fluoresce., 2015, vol. 25, pp. 1727–1738. https://doi.org/10.1007/s10895-015-1659-1
Salotra, R. and Utreja, D., Curr. Org. Chem., 2020, vol. 24, pp. 2755–2781. https://doi.org/10.2174/1385272824999200922090524
Saglam, M.F., Bingul, M., Şenkuytu, E., Boga, M., Zorlu, Y., Kandemir, H., and Sengul, I.F., J. Mol. Struct., 2020, vol. 1215, p. 128308. https://doi.org/10.1016/j.molstruc.2020.128308
Rathod, A.S., Reddy, P.V., and Biradar, J.S., Russ. J. Org. Chem., 2020, vol. 56, pp. 662–670. https://doi.org/10.1134/S1070428020040156
Rahim, F., Taha, M., Ullah, H., Wadood, A., Selvaraj, M., Rab, A., Sajid, M., Shah, S.A., Uddin, N., and Gollapalli, M., Bioorg. Chem., 2019, vol. 91, p. 103112. https://doi.org/10.1016/j.bioorg.2019.103112
Fang, Z., Yan, J., Yu, W., Zhang, N., and Zhang, S., Transition Met. Chem., 2019, vol. 44, pp. 463–474. https://doi.org/10.1007/s11243-019-00327-1
Feng, Y., Feng, L., Sun, Y., and He, J., J. Mater. Res. Technol., 2020, vol. 9, pp. 584–593. https://doi.org/10.1016/j.jmrt.2019.10.087
Das, P.K., Sahu, R., Satapathy, P., Dash, D., and Garnaik, B., Asian J. Chem., 2018, vol. 30, pp. 556–560. https://doi.org/10.14233/ajchem.2018.20986
Kaur, L., Utreja, D., and Dhillon, N.K., Russ. J. Org. Chem., 2021, vol. 57, pp. 961–967. https://doi.org/10.1134/S1070428021060129
Jain, P., Utreja, D., and Sharma, P., J. Heterocycl. Chem., 2020, vol. 57, pp. 428–435. https://doi.org/10.1002/jhet.3799
Ramesh, D., Joji, A., Vijayakumar, B.G., Sethumadhavan, A., Mani, M., and Kannan, T., Eur. J. Med. Chem., 2020, vol. 198, p. 112358. https://doi.org/10.1016/j.ejmech.2020.112358
Ejidike, I.P. and Ajibade, P.A., Rev. Inorg. Chem., 2015, vol. 35, pp. 191–224. https://doi.org/10.1515/revic-2015-0007
Mohan, C., Kumar, V., Kumari, N., Kumari, S., Yadav, J., Gandass, T., and Yadav, S., Adv. J. Chem. B, 2020, vol. 2, pp. 187–196. https://doi.org/10.22034/AJCB.2020.113663
Taha, M., Ismail, N.H., Javaid, K., Imran, S., Wadood, A., Ali, M., Khan, K.M., Saad, S.M., Rahim, F., and Choudhary, M.I., Bioorg. Chem., 2015, vol. 1, pp. 24–35. https://doi.org/10.1016/j.bioorg.2015.09.001
Kaur, K., Utreja, D., Dhillon, N.K., Pathak, R.K., and Singh, K., Pest. Biochem. Physiol., 2021, vol. 171, p. 104736. https://doi.org/10.1016/j.pestbp.2020.104736
Goyal, A., Utreja, D., Garg, A., and Sharma, V.K., Agri. Res. J., 2018, vol. 55, pp. 377–379. https://doi.org/10.5958/2395-146X.2018.00070.4
Dawar, M., Utreja, D., Rani, R., and Kaur, K., Lett. Org. Chem., 2020, vol. 17, pp. 199–205. https://doi.org/10.2174/1570178616666190724120308
Kumbar, B., Mahmood, R., Nagesha, S.N., Nagaraja, M.S., Prashant, D.G., Kerima, O.Z., Karosiya, A., and Chavan, M., Biocatal. Agric. Biotechnol., 2019, vol. 22, p. 101366. https://doi.org/10.1016/j.bcab.2019.101366
McGovern, R.J., Crop Prot., 2015, vol. 73, pp. 78–92. https://doi.org/10.1016/j.cropro.2015.02.021
Salotra, R., Utreja, D., and Sharma, P.A., Russ. J. Org. Chem., 2020, vol. 56, pp. 2207–2211. https://doi.org/10.1134/S1070428020120258
Kaur, J., Utreja, D., Dhillon, N.K., and Sharma, S., Lett. Org. Chem., 2019, vol. 16, pp. 759–767. https://doi.org/10.2174/1570178616666190219131042
Haney, E.F., Trimble, M.J., Cheng, J.T., Vallé, Q., and Hancock, R.E., Biomolecules, 2018, vol. 8, pp. 29. https://doi.org/10.3390/biom8020029
El-Sawy, E., Abo-Salem, H., and Mandour, A., Egypt. J. Chem., 2017, vol. 60, pp. 723–751. https://doi.org/10.21608/ejchem.2017.1097.1053
Ali, I., Mukhtar, S.D., Hsieh, M.F., Alothman, Z.A., and Alwarthan, A., RSC Adv., 2018, vol. 8, pp. 37905–37914. https://doi.org/10.1039/C8RA07060A
Espinel-Ingroff, A., Kerkering, T.M., Goldson, P.R., and Shadomy, S., J. Clin. Microbiol., 1991, vol. 29, pp. 1089–1094. https://doi.org/10.1128/jcm.29.2.393-394.1991
Sridhar, S.R., Rajagopal, R.V., Rajavel, R., Masilamani, S., and Narasimhan, S., J. Agric. Food Chem., 2003, vol. 51, pp. 7596–7599. https://doi.org/10.1021/jf0344082
ACKNOWLEDGMENTS
We gratefully acknowledge Punjab Government (section P-5B-1972) and Sophisticated Analytical Instrumentation Facility, Panjab University, Chandigarh for analysis of compounds reported in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Priya, B., Utreja, D. & Kalia, A. Schiff Bases of Indole-3-Carbaldehyde: Synthesis and Evaluation as Antimicrobial Agents. Russ J Bioorg Chem 48, 1282–1290 (2022). https://doi.org/10.1134/S1068162022060188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022060188