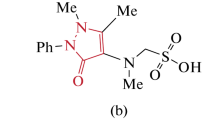
Abstract
In this study, some Schiff bases derived from 4-aminoantipyrine (4-AAP) (VI–X) were synthesized, characterized by elemental analysis (C, H, N) and three spectral techniques (FT-IR, 1H, and 13C NMR), and then their antioxidant activity was investigated by employing four different methods. Subsequently, the inhibitory influences of the synthesized molecules against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase enzymes were tested. More importantly, the cytotoxic effects of all title molecules were also evaluated on HeLa human cervical cancer and L929 mouse fibroblast cell lines. According to the results obtained, compound (VI) (IC50: 16.82 ± 0.17 μM) showed higher ABTS cation radical scavenging activity than BHA (IC50: 17.59 ± 0.10 μM). In CUPRAC assay, it was determined that the activity ordering of the bioactive molecules has been determined as VII > X > VII > α-TOC > IX > I > II > V > VI. In AChE assay, compound (I) indicated a high potent inhibition activity with 91.75 ± 1.15% better than galanthamine. In BChE assay, compound (VII) had good BChE inhibition activity (78.76 ± 1.47%) better than galanthamine. Compound (V) showed strong tyrosinase inhibition activity with 52.85 ± 0.23% value at 200 μM concentration. Compound (III) showed the best cytotoxic effect on HeLa cells with an IC50 dose of 21.47 µM. Consequently, it can be said that some of these synthesized molecules were potentially new anti-Alzheimer drug candidate molecules.



Similar content being viewed by others
REFERENCES
Salamizadeh, A., Mirzaei, T., and Ravari, A., Int. J. Commun. Based Nurs. Midwifery, 2017, vol. 5, pp. 231–238.
Maresova, P., Mohelská, H., Dolejs, J., and Kuca, K., Curr. Alzheimer Res., 2015, vol. 12, pp. 903–911. https://doi.org/10.2174/156720501209151019111448
Monroe, T., Carter, M., Feldt, K., Tolley, B., and Cowan, R.L., J. Adv. Nurs., 2012, vol. 68, pp. 2070–2078. https://doi.org/10.1111/j.1365-2648.2011.05929.x
Bray, F., Laversanne, M., Weiderpass, E., and Soerjomataram, I., Cancer, 2021, vol. 127, pp. 3029–3030. https://doi.org/10.1002/cncr.33587
Al-Mulla, A., Pharma Chem., 2017, vol. 9, pp. 141–147.
Aljamali, N.M., Asian J. Chem., 2014, vol. 7, pp. 975–1006.
Zhang, Z., and Zaworotko, M.J., Chem. Soc. Rev., 2014, vol. 43, pp. 5444–5455. https://doi.org/10.1039/C4CS00075G
Adeniji, S.E., Arthur. D.E., Abdullahi. M., and Adalumo, O.B., J. Bull. Natl. Res. Cent., 2020, vol. 44, pp. 1–17. https://doi.org/10.1186/s42269-020-00386-w
Shanty, A.A., Philip, J.E., Sneha, E. J., Kurup, M.P., Balachandran, S., and Mohanan, P.V., Bioorg. Chem., 2017, vol. 70, pp. 67–73. https://doi.org/10.1016/j.bioorg.2016.11.009
Ouerghi, O., Geesi, M.H., Kaiba, A., Al-Tamimi, A.M.S., Guionneau, P., Ibnouf, E.O., Azzallou, R., Bakht, M.A., and Riadi, Y., J. Mol. Struct., 2021, vol. 1225, p. 129166. https://doi.org/10.1016/j.molstruc.2020.129166
Alam, M. S., and Lee. D.U., J. Mol. Struct., 2021, vol. 1227, p. 129512. https://doi.org/10.1016/j.molstruc.2020.129512
Okey, N.C., Obasi, N. L., Ejikeme, P.M., Ndinteh, D.T., Ramasami, P., Sherif, E.S.M., Akpan, E.D., and Ebenso, E.E., J. Mol. Liq., 2020, vol. 315, p. 113773. https://doi.org/10.1016/j.molliq.2020.113773
Upadhyay, A., Kar, P.K., and Dash, S., Spectrochim. Acta A Mol. Biomol. Spectrosc., 2020, vol. 233, p. 118231. https://doi.org/10.1016/j.saa.2020.118231
Sakthivel, A., Jeyasubramanian, K., Thangagiri, B., Raja, J.D., J. Mol. Struct., 2020, vol. 1222, p. 128885. https://doi.org/10.1016/j.molstruc.2020.128885
Fathima, S.S.A., Paulpandiyan, R., and Nagarajan, E.R., J. Mol. Struct., 2019, vol. 1178, pp. 179–191. https://doi.org/10.1016/j.molstruc.2018.10.021
Teran, R., Guevara, R., Mora, J., Dobronski, L., Barreiro-Costa, O., Beske, T., Pérez-Barrera, J., Araya-Maturana, R., Rojas-Silva, P., Povedo, A., and Heredia-Moya, J., Molecules., 2019, vol. 24, p. 2696. https://doi.org/10.3390/molecules24152696
Raman, N., Mitu, L., Sakthivel, A., and Pandi, M.S.S., J. Iran. Chem. Soc., 2009, vol. 6, pp. 738–748. https://doi.org/10.1007/BF03246164
Ocheni, A. and Clement, U., Chem. Int., 2017, vol. 3, pp. 244–249.
Tok, F., Koçyiğit-Kaymakçıoğlu, B., Sağlık, B.N., Levent, S., Özkay, Y., and Kaplancıklı, Z.A., Bioorg. Chem., 2019, vol. 84, pp. 41–50. https://doi.org/10.1016/j.bioorg.2018.11.016
Kolcu, F., Erdener, D., and Kaya, İ., Synth. Meth., 2021, vol. 272, p. 116668. https://doi.org/10.1016/j.synthmet.2020.116668
Urasa, M., and Darj, E., Afr. Health Sci., 2011, vol. 11 pp. 48–57.
Mauricio, D., Zeybek, B., Tymon-Rosario, J., Harold, J., and Santin, A. D., Curr. Oncol. Rep., 2021, vol. 23, pp. 1–12. https://doi.org/10.1007/s11912-021-01052-8
Kim, H.S., Park, S.Y., Park, C.Y., Kim, Y.T., Kim, B.J., Song, Y.J., Kim, B.G., Kim, Y.B., Cho, C.H., Kim, J.H., and Song, Y.S., Br. J. Cancer, 2021, vol. 124, pp. 375–382. https://doi.org/10.1038/s41416-020-01098-8
Hirte, H., Poon, R., Yao, X., May, T., Ethier, J.L., Petz, L., Speakman, J., and Elit, L., Crit. Rev. Oncol. Hematol., 2021, vol. 162, p. 103324. https://doi.org/10.1016/j.critrevonc.2021.103324
Zhang, H., Xu, H., Ashby, C.R., Jr., Assaraf, Y.G., Chen, Z.S., and Liu, H.M., Med. Res. Rev., 2021, vol. 41, pp. 525–555. https://doi.org/10.1002/med.21739
Chen, H., Wang, T., Huang, S., and Zeng, P., J. Cancer, 2021, vol. 12, pp. 840–848.
Fang, Y.Z., Yang, S., and Wu, G., Nutrition, 2002, vol. 18, pp. 872–879. https://doi.org/10.1016/S0899-9007(02)00916-4
Bast, A., and Goris, R. J. A., Weekbl. Sci., 1989, vol. 11, pp. 199–206.
Baublis, A.J., Clydesdale, F.M., and Decker, E.A., Cereal Foods World, 2000, vol. 45, pp. 71–74.
Keren-Shaul, H., Spinrad, A., Weiner, A., Matcovitch-Natan, O., Dvir-Szternfeld, R., Ulland, T.K., David, E., Baruch, K., Lara-Astaiso, D., Toth, B., Itzkovitz, S., Colonna, M., Schwart, M., and Amit, I., Cell, 2017, vol. 169, pp. 1276–1290. https://doi.org/10.1016/j.cell.2017.05.018
Möller, H.J., and Graeber, M.B., Eur. Arch. Psychiatry Clin. Neurosci., 1998, vol. 248, pp. 111–122. https://doi.org/10.1007/s004060050027
Maurer, K., Volk, S., and Gerbaldo, H., Lancet, 1997, vol. 349, pp. 1546–1549.
Coyle, J.T., Price, D.L., and Delong, M.R., Science, 1983, vol. 219, pp. 1184–1190. https://doi.org/10.1126/science.6338589
Pohanka, M., Bratisl. Lek. Listy, 2018, vol. 119, pp. 535–543.
Klugman, A., Naughton, D.P., Isaac, M., Shah, I., Petroczi, A., and Tabet, N., J. Alzheimer’s Dis., 2012, vol. 30, pp. 467–474. https://doi.org/10.3233/JAD-2012-120124
Farlow, M. R. Int. J. Clin. Pract. Suppl., 2002, vol. 127, pp. 37–44.
Das, U. N., Ann. Hepatol., 2012, vol. 11, pp. 409–411.
Jope, R.S., Walter-Ryan, W.G., Alarcon, R.D., and Lally, K.M., Biol. Psychiatry, 1985, vol. 20, pp. 1258–1266. https://doi.org/10.1016/0006-3223(85)90110-6
Zolghadri, S., Bahrami, A., Hassan Khan, M.T., Munoz-Munoz, J., Garcia-Molina, F., Garcia-Canovas, F., and Saboury, A.A., Enzyme Inhib. Med. Chem., 2019, vol. 34, pp. 279–309. https://doi.org/10.1080/14756366.2018.1545767
Topal, G., Tombak, A., Yigitalp, E., Batibay, D., Kilicoglu, T., and Ocak, Y.S., J. Electron. Mater., 2017, vol. 46, p. 3958. https://doi.org/10.1007/s11664-017-5446-4
Çınar, E., Başaran, E., Erdoğan, Ö., Çakmak, R., Boğa, M., and Çevik, Ö., J. Chin. Chem. Soc., 2021, pp. 1–13. https://doi.org/10.1002/jccs.202100357
Paşa, S., Erdoğan, Ö., and Yenisey, Ç., J. Mol. Struct., 2019, vol. 1186, pp. 458–467. https://doi.org/10.1016/j.molstruc.2019.03.061
Premnath, D., Selvakumar, P.M., Ravichandiran, P., Selvan, G.T., Indiraleka, M., Vennila., J.J., Spectrochim. Acta, Part A: Mol. Biomol. Spectrosc., 2016, vol. 153, pp. 118–123. https://doi.org/10.1016/j.saa.2015.08.008
Raiford, L.C. and Milbery, J.E., J. Am. Chem. Soc., 1934, vol. 56, pp. 2727–2729. https://doi.org/10.1021/ja01327a060
Harris, C.M., Hobson, A.D., and Wilson, N.S., US Patent Application No. 12/703615, 2010.
Hu, P., Zhao, K.Q., Xu, H.B., and Zhang, L.F., Chem. J. Chin. Univ., 2003, vol. 24, pp. 2195–2201.
Um, I.H., Han, H.J., Ahn, J.A., Kang, S., and Buncel, E., J. Org. Chem., 2002, vol. 67, pp. 8475–8480. https://doi.org/10.1021/jo026339g
Blois, M.S., Nature, 1958, vol. 18, pp. 1199–1200.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C., Free Radicals Biol. Med., 1999, vol. 26, pp. 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Dinis, T.C., Madeira, V.M., and Almeida, L.M., Arch. Biochem., 1994, vol. 315, pp. 161–169. https://doi.org/10.1006/abbi.1994.1485
Apak, R., Güçlü, K., Özyürek, M., and Karademir, S.E., J. Agric. Sci., 2004, vol. 52, pp. 7970–7981. https://doi.org/10.1021/jf048741x
Hearing, V. J. and Jiménez, M., Int. J. Biochem., 1987, vol. 19, pp. 1141–1147. https://doi.org/10.1016/0020-711X(87)90095-4
Ellman, G.L., Courtney, K.D., Andres, V., Jr., and Featherstone, R.M., Biochem. Pharmacol., 1961, vol. 7, pp. 88–90. https://doi.org/10.1016/0006-2952(61)90145-9
Bingul, M., Ercan, S., and Boğa, M., J. Mol. Struct., 2020, vol. 1213, p. 128202. https://doi.org/10.1016/j.molstruc.2020.128202
Erdoğan, O., Abbak, M., Demirbolat, G.M., Birtekocak, F., Aksel, M., Paşa, S., and Cevik, O., PLoS One, 2019, vol. 14, art. e0216496. https://doi.org/10.1371/journal.pone.0216496
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving human participants performed by any of the authors and does not contain any studies involving animals performed by any of the author.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Additional information
Corresponding autor: phone: +90 488 217 35 94.
Supplementary Information
Rights and permissions
About this article
Cite this article
Reşit Çakmak, Başaran, E., Boğa, M. et al. Schiff Base Derivatives of 4-Aminoantipyrine as Promising Molecules: Synthesis, Structural Characterization, and Biological Activities. Russ J Bioorg Chem 48, 334–344 (2022). https://doi.org/10.1134/S1068162022020182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022020182