Abstract—
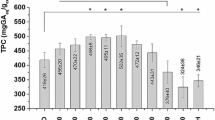
The isolation of proanthocyanidins from the bark of Scotch pine Pinus sylvestris L. by water and water–alcohol solutions containing 5, 10, 15, 20, and 25% ethanol was studied for the first time. Isolation of proanthocyanidins was carried out from the initial and deresined (extracted with hexane) pine bark. It was shown that, compared with water extraction, the use of 15–25% aqueous ethanol solutions allows one to increase the yield of proanthocyanidins from 0.44 to 0.63%. It was established that the preliminary extraction of resinous substances from the pine bark does not significantly affect the yield of proanthocyanidins. It was shown that an increase in ethanol concentration of more than 20% in the extraction solution leads to an increase in the total yield of extractives, while the yield of proanthocyanidins does not increase. A study of proanthocyanidins by UV spectroscopy after their conversion to red anthocyanidins showed that they mainly consist of procyanidin and prodelphinidine in close concentrations. The composition of the proanthocyanidins mixtures obtained was studied by IR and 13C NMR spectroscopy. It was shown that the proanthocyanidins obtained from the bark of pine Pinus sylvestris L., in contrast to isolated from other pine species, contains gallic acid residues which can increase their antiradical activity.



Similar content being viewed by others
REFERENCES
Sprygin, V.G. and Kushnerova, N.F., Cranberry: A new source of oligomeric proanthocyanidins, Pharm. Chem. J., 2004, vol. 38, no. 2, pp. 100–104. https://doi.org/10.1023/B:PHAC.0000032489.09856.52
Santos-Buelga, C. and Scalbert, A., Proanthocyanidins and tannin-like compounds—nature, occurrence, dietary intake and effects on nutrition and health, J. Sci. Food Agric., 2000, vol. 80, no. 7, pp. 1094–1117. https://doi.org/10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1
Osipov, V.I., Polyakov, N.A., Sidel’nikov, A.N., an Khazieva, F.M., Proanthocyanidins of roots and rhizomes of Potentilla alba (Rosaceae), Rastit. Resur., 2017, vol. 53, no. 1, pp. 114–125.
Ossipova, S., Ossipov, V., Haukioja, E., Loponen, J., and Pihlaja, K., Proanthocyanidins of mountain birch leaves: quantification and properties, Phytochem. Anal., 2001, vol. 12, no. 2, pp. 128–133. https://doi.org/10.1002/pca.568
Karonen, M., Leikas, A., Loponen, J., Sinkkonen, J., Ossipov, V., and Pihlaja, K., Reversed-phase HPLC-ESI/MS analysis of birch leaf proanthocyanidins after their acidic degradation in the presence of nucleophiles, Phytochem. Anal., 2007, vol. 18, no. 5, pp. 378–386. https://doi.org/10.1002/pca.992
Bors, W., Michel, C., and Stettmaier, K., Electron paramagnetic resonance studies of radical species of proanthocyanidins and gallate esters, Arch. Biochem. Biophys., 2000, vol. 374, no. 2, pp. 347–355. https://doi.org/10.1006/abbi.1999.1606
Renaud, S. and Gueguen, R., The French paradox and wine drinking, Novartis Found. Symp., 1998, vol. 216, pp. 208–217. https://doi.org/10.1002/9780470515549.ch13
Orgogozo, J.M., Dartigues, J.F., Lafont, S., Letenneur, L., Commenges, D., Salamon, R., Renaud, S., and Breteler, M.B., Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area, Rev. Neurol. (Paris), 1997, vol. 153, no. 3, pp. 185–192.
Stabgler, HerodezS., Hadolin, M., Skerget, M., Perva, A., Knez, Z., and Bauman, D., Isolation of proanthocyanidins from different natural sources, Chem. Ing. Tech., 2001, vol. 73, no. 6, pp. 731–731. https://doi.org/10.1002/1522-2640(200106)73:6<731::AID-CITE7313333>3.0.CO;2-O
Sprygin, V.G. and Kushnerova, N.F., A method for evaluation and standardization of oligomeric proanthocyanidin complexes isolated from various raw plant materials, Pharm. Chem. J., 2002, vol. 36, no. 3, pp. 139–143. doi https://doi.org/10.1023/A:1019682311646
Chang, Q., Zhu, M., Zuo, Z., Chow, M., and Ho, W.K.K., High-performance liquid chromatographic method for simultaneous determination of hawthorn active components in rat plasma, J. Chromatogr. B: Biomed. Sci. Appl., 2001, vol. 760, no. 2, pp. 227–235. https://doi.org/10.1016/S0378-4347(01)00273-0
Kim, S.H., Kang, K.W., Kim, K.W., and Kim, N.D., Procyanidins in Crataegus extract evoke endothelium-dependent vasorelaxation in rat aorta, Life Sci., 2000, vol. 67, no. 2, pp. 121–131. https://doi.org/10.1016/S0024-3205(00)00608-1
Ku, C.S. and Mun, S.P., Characterization of proanthocyanidin in hot water extract isolated from Pinus radiata bark, Wood Sci. Technol., 2007, vol. 41, no. 3, pp. 235–247. https://doi.org/10.1007/s00226-006-0103-8
Yazaki, Y., Utilization of flavonoid compounds from bark and wood: a review, Nat. Prod. Commun., 2015, vol. 10, no. 3, pp. 513–520. https://doi.org/10.1177/1934578X1501000333
Levdanskii, V.A., Butylkina, A.I., Levdanskii, A.V., and Kuznetsov, B.N., A method of obtaining proanthocyanidins from Scotch pine bark, RF Patent no. 2375070, 2009.
Deineko, I.P. and Faustova, N.M., Element and group composition of aspen bark and wood, Khim. Rastit. Syr’ya, 2015, no. 1, pp. 51–62. https://doi.org/10.14258/jcprm.201501461
Diouf, P.N., Tibirna, C.M., Garcia-Perez, M.-E., Royer, M., Dube, P., and Stevanovic, T., Structural elucidation of condensed tannin from Picea mariana bark, JBNB, 2013, vol. 4, no. 3A, pp. 1–8. https://doi.org/10.4236/jbnb.2013.43A001
Fu, C., Yang, D., Peh, W.Y.E., Lai, S., Feng, X., and Yang, H., Structure and antioxidant activities of proanthocyanidins from elephant apple (Dillenia indica Linn.), J. Food Sci., 2015, vol. 80, no. 10, pp. C2191–C2199. https://doi.org/10.1111/1750-3841.13005
Plumb, G.W., De Pascual-Teresa, S., Santos-Buelga, C., Cheynier, V., and Williamson, G., Antioxidant properties of catechins and proanthocyanidins: effect of polymerisation, galloylation and glycosylation, Free Radical Res., 1998, vol. 29, no. 4, pp. 351–358. https://doi.org/10.1080/10715769800300391
Funding
The work was performed within the framework of the state assignment of the Institute of Chemical Chemistry of the SB RAS, project AAAA-A17-117021310219-4 (V.46.4.2.) Using the equipment of the Krasnoyarsk Regional Center for Collective Use of the Federal Research Center of the KSC SB RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Levdanskiy, V.A., Korol’kova, I.V., Levdanskiy, A.V. et al. Isolation and Study of Proanthocyanidins from Bark of Pine Pinus sylvestris L.. Russ J Bioorg Chem 47, 1445–1450 (2021). https://doi.org/10.1134/S1068162021070098
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021070098