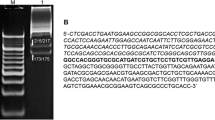
Abstract
From the Bacillus species D6 strain, the restriction endonuclease BspD6II has been isolated and characterized. It recognizes the asymmetric region 5′-CTGAAG-3′/3′-GACTTC-5′ in the double-stranded DNA. The sites of DNA hydrolysis by this enzyme have been determined. They are localized beyond the recognition site 16 nt downstream of the sequence 5′-CTGAAG-3′ toward the 3′-end of DNA and 14 nt toward the 5′-end in the opposite strand. It has been shown that BspD6II differs in properties from isoschizomer enzymes Eco57I (prototype), AcuI, and BspKT5I and has some advantages. It catalyzes the exhaustive hydrolysis of DNA in the absence of S-adenosyl-L-methionine and effectively operates at 50°C, i.e., is a thermophilic enzyme.
Similar content being viewed by others
Abbreviations
- DTT:
-
dithiothreitol
- Met(Ado):
-
S-adenosyl-Lmethionine
- nt:
-
nucleotide
- NE:
-
nicking endonuclease
- RMS:
-
restriction-modification system
- RE:
-
restriction endonuclease
References
Zheleznaya, L.A., Perevyazova, T.A., Alzhanova, D.V., and Matvienko, N.I., Biochemistry (Moscow), 2001, vol. 66, no. 3, pp. 989–993.
Yunusova, A.K., Rogulin, E.A., Artyukh, R.I., Zheleznaya, L.A., and Matvienko, N.I., Biochemistry (Moscow), 2006, vol. 71, no. 7, pp. 815–820.
Kachalova, G.S., Rogulin, E.A., Yunusova, A.K., Artyukh, R.I., Perevyazova, T.A., Matvienko, N.I., Zheleznaya, L.A., and Bartunik, H.D., J. Mol. Biol., 2008, vol. 384, pp. 489–502.
Abrosimova, L.A., Kubareva, E.A., Migur, A.Y., Gavshina, A.V., Ryazanova, A.Y., Norkin, M.V., Perevyazova, T.A., Wende, W., Hianik, T., Zheleznaya, L.A., and Oretskaya, T.S., Biochim. Biophys. Acta, Proteins Proteomics, 2016, vol. 1864, pp. 1072–1082.
Petrusyte, M., Bitinaite, J., Menkevicius, S., Klimasauskas, S., Butkus, V., and Janulaitis, A.A., Gene, 1988, vol. 72, pp. 89–91.
Janulailis, A., Petrusyte, M., Maneliene, Z., Klimasauskas, S., and Burkus, V., Nucleic Acids Res., 1992, vol. 20, pp. 6043–6049.
Brown, N.L. and Smith, M., Methods Enzymol., 1980, vol. 65, pp. 391–404.
Zheleznaya, L.A., Fedorov, O.V., Brik, A.F., and Matvienko, N.I., Biokhimiya, 1994, vol. 59, pp. 1621–1637.
Sanger, F. and Coulson, A., J. Mol. Biol., 1975, vol. 94, pp. 444–448.
Samuelson, J., Xu, S.-Y., and O’Loane, D., US Patent no. 7011966.
Shapovalova, N.I., Zheleznaya, L.A., Matvienko, N.N., and Matvienko, N.I., Biokhimiya, 1994, vol. 59, pp. 485–493.
Roberts, R.J. and Macels, D., Nucleic Acids Res., 2003, vol. 29, pp. 268–269.
Wingfield, P.T., Curr. Protoc. Protein Sci., 2016, vol. 84, pp. 1–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.A. Perevyazova, A.K. Yunusova, R.I. Artyukh, M.B. Viryasov, E.A. Kubareva, L.A. Zheleznaya, 2018, published in Bioorganicheskaya Khimiya, 2018, Vol. 44, No. 6, pp. 648–654.
Rights and permissions
About this article
Cite this article
Perevyazova, T.A., Yunusova, A.K., Artyukh, R.I. et al. Restriction Endonuclease BspD6II, a New Thermophilic Isoschizomer of Eco57I. Russ J Bioorg Chem 44, 681–686 (2018). https://doi.org/10.1134/S1068162018040143
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162018040143