Abstract
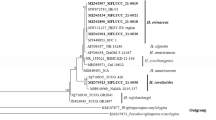
The activity of extracellular polysaccharide-degrading enzymes and glycosidases from mycelial fungi towards various carbohydrates and carbohydrate derivatives from plant and algal cell walls has been screened. Twenty-three strains of mycelial fungi isolated from the marine sediment and dung were grown by submerged cultivation on a plant-based substrate (a by-product of the grain processing industry) for previous screening for their biomass and protein productivity. Molecular identification allowed for the assignment of marine fungal strains to the following species: Sirastachys phyllophila, Ochroconis mirabilis, Pseudallescheria boydii, Pseudallescheria ellipsoidea, Beauveria felina, Scopulariopsis brevicaulis, Cladosporium sp., and Trichoderma sp. The terrestrial strains belonged to the species Thermomyces thermophilus, Thermomyces dupontii, Thermomyces lanuginosus, Fusarium avenaceum, Mycothermus thermophilum, and Thermothelomyces thermophila. Seven strains of thermophilic terrestrial fungal species T. thermophila, T. thermophilus, T. dupontii and M. thermophilus and two marine fungal strains of S. brevicaulis and Beauveria felina exhibited the highest protein yields and a wide range of polysaccharide-degrading activity when the cultures were cultivated at 22–25°C. The cellulolytic thermophilic strain M. thermophilus 55 isolated from dung demonstrated unusual specificity, most intensive increase of mycelial biomass, and high activity towards algal polysaccharides after seven days of cultivation. The specific activity of laminarinase was one order of magnitude higher than in the marine strains and amounted to 1180 U/mg, and the alginate lyase, carrageenase, polymannuronate lyase, agarase, and fucoidanase activity levels (from 208 to 500 U/mg) were also higher than in all marine strains. All active polysaccharide-degrading strains of thermophilic terrestrial and marine fungi identified in the present study are of considerable interest, as the potential of these fungi for polysaccharide degradation can be applied in the transformation of various agricultural and maricultural waste of plant origin and in the modification of carbohydrate-containing substances in structural research and biotechnology.
Similar content being viewed by others
References
Suryanarayanan, T.S., Venkatachalam, A., Thirunavukkarasu, N., Ravishankar, J.P., Doble, M., and Geetha, V., Bot. Mar., 2010, vol. 53, pp. 457–468.
Kumar, A., Henrissat, B., Arvas, M., Syed, M.F., Thieme, N., Benz, J.P., Sorensen, J.L., Record, E., Pöggeler, S., and Kempken, F., PLoS One, 2015, vol. 10. e0140398.
Le Calvez, T., Burgaud, G., Mahe, S., Barbier, G., and Vandenkoornhuyse, P., Appl. Environ. Microbiol., 2009, vol. 75, pp. 6415–6421.
Richards, T.A., Leonard, G., Mahe, F., del Campo, J., Romac, S., Jones, M.D.M., Maguire, F., Dunthorn, M., De Vargas, C., and Massana, R., Proc. Biol. Sci., 2015, vol. 282, pp. 2015–2243.
Sobolevskaya, M.P., Leshchenko, E.V., Hoai, T.P.T., Denisenko, V.A., Dyshlovoy, S.A., Kirichuk, N.N., Khudyakova, Y.V., Kim, N.Yu., Berdyshev, D.V., and Pislyagin, E.A., J. Nat. Prod., 2016, vol. 79, pp. 3031–3038.
Oleinikova, G.K., Denisenko, V.A., Berdyshev, D.V., Pushilin, M.A., Kirichuk, N.N., Menzorova, N.I., Kuzmich, A.S., Yurchenko, E.A., Zhuravleva, O.I., and Afiyatullov, Sh., Phytochem. Lett., 2016, vol. 17, pp. 135–139.
Levasseur, A., Drula, E., Lombard, V., Coutinho, P.M., and Henrissat, B., Biotechnol. Biofuels, 2013, vol. 6, p. 41. doi.org/doi 10.1186/1754-6834–6–41
Hooley, P. and Whitehead, M., Mycologist, 2006, vol. 20, pp. 144–151.
Brink, J. and de Vries, R.P., Appl. Microbiol. Biotechnol., 2011, vol. 91, pp. 1477–1492.
Aro, N., Pakula, T., and Penttila, M., FEMS Microbiol. Rev., 2005, vol. 29, pp. 719–739.
Synytsya, A., Čopíková, J., Kim, W.J., and Park, Y.I.I., in Springer Handbook in Marine Biotechnology, Kim, S.-K., Ed., Mumbai: Springer-Verlag, 2015, pp. 543–590.
Deniaud-Boue, E., Kervarec, N., Michel, G., Tonon, T., Kloareg, B., and Herve, C., Ann. Bot., 2014, vol. 14, pp. 1203–1216.
Behera, S., Singh, R., Arora, R., Sharma, N.K., Shukla, M., and Kumar, S., Front. Bioeng. Biotechnol., 2015, vol. 2, p. 90. doi 10.3389/fbioe.2014.00090
Ulaganathan, T., Boniecki, M.T., Foran, E., Buravenkov, V., Mizrachi, N., Banin, E., Helbert, W., and Cygler, M., ACS Chem. Biol., 2017, vol. 12, pp. 1269–1280.
Kusaykin, M.I., Silchenko, A.S., Zakharenko, A.M., and Zvyagintseva, T.N., Glycobiology, 2016, vol. 26, pp. 1–3.
Pluvinage, B., Hehemann, J.-H., and Boraston, A.B., J. Biol. Chem., 2013, vol. 288, pp. 28078–28088.
Gao, B., Jin, M., Li, L., Qu, W., and Zeng, R., Front. Microbiol., 2017, vol. 8, p. 600. doi 10.3389/fmicb.2017.00600
Zhu, Y., Chen, P., Bao, Y., Men, Y., Zeng, Y., Yang, J., Sun, J., and Suna, Y., Sci. Rep., 2016, vol. 6, p. 38248.
Wang, Y., Barth, D., Tamminen, A., and Wiebe, M.G., BMC Biotechnol., 2016, vol. 16, p. 3. doi.org/0.1186/s12896–016–0233–5
Trivedi, N., Reddy, C., Radulovich, R., and Jha, B., Algal Res., 2015, vol. 9, pp. 48–54.
Sathya, R. and Ushadevy, T., Indian J. Appl. Res., 2013, vol. 3, pp. 308–309.
Dhale, M.A. and Vijay-Raj, A.S., Int. J. Food Sci. Technol., 2009, vol. 44, pp. 2424–2430.
Karnaouri, A., Topakas, E., Antonopoulou, I., and Christakopoulos, P., Front. Microbiol., 2014, vol. 5, p. 281. doi 10.3389/fmicb.2014.00281
Douglas, C.M., Med. Mycol., 2001, vol. 39, pp. 55–66.
Burtseva, Yu.V., Sova, V.V., Pivkin, M.V., Anastyuk, S.D., Gorbach, V.I., and Zvyagintseva, T.N., Appl. Biochem. Microbiol., 2010, vol. 46, pp. 648–656.
Bakunina, I., Nedashkovskaya, O., Balabanova, L., Zvyagintseva, T., Rasskasov, V., and Mikhailov, V., Mar. Drugs, 2013, vol. 11, pp. 1977–1998.
Bilai, V.I., Metody eksperimental’noi mikologii (Methods of Experimental Mycology), Kiev: Naukova dumka, 1982.
Bilai, T.I. and Zakharchenko, V.A., Opredelitel’ termofil’nykh gribov (Identification Guide to Thermophilic Fungi), Kiev: Naukova dumka, 1987.
Egorova, L.N., Pochvennye griby Dal’nego Vostoka: Gifomitsety (Soil Fungi of the Far East: Hyphomycetes), Leningrad: Nauka, 1986.
Litvinov, M.A., Opredelitel’ mikroskopicheskikh pochvennykh gribov (Identification Guide to Microscopic Soil Fungi), Leningrad: Nauka, 1967.
Bredford, M.M., Anal. Biochem., 1976, vol. 72, pp. 248–254.
Nelson, T.E., J. Biol. Chem., 1944, vol. 153, pp. 375–381.
Urvantseva, A.M., Bakunina, I.Yu., Kim, N.Yu., Isakov, V.V., Glazunov, V.P., and Zvyagintseva, T.N., Khim. Rastit. Syr’ya, 2004, vol. 3, pp. 15–24.
Kusaykin, M.I., Bakunina, I.Y., Sova, V.V., Ermakova, S.P., Kuznetsova, T.S., Besednova, N.N., Zaporozhets, T.S., and Zvyagintseva, T.N., J. Biotechnol., 2008, vol. 3, pp. 904–915.
Zvyagintseva, T.N., Shevchenko, N.M., and Nazarenko, E.L., J. Exp. Mar. Biol. Ecol., 2005, vol. 320, pp. 123–131.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.A. Balabanova, I.Yu. Bakunina, L.V. Slepchenko, N.N. Kirichuk, Yu.V. Khudyakova, O.M. Son, M.V. Pivkin, V.A. Rasskazov, 2018, published in Bioorganicheskaya Khimiya, 2018, Vol. 44, No. 4, pp. 425–432.
The article is based on materials presented as a communication at the Second Elyakov Scientific Conference in Vladivostok on October 6, 2017.
Deceased.
Rights and permissions
About this article
Cite this article
Balabanova, L.A., Bakunina, I.Y., Slepchenko, L.V. et al. Polysaccharide-Degrading Activity in Marine and Terrestrial Strains of Mycelial Fungi. Russ J Bioorg Chem 44, 431–437 (2018). https://doi.org/10.1134/S1068162018040039
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162018040039