Abstract
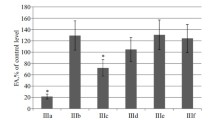
Protein glycation is believed to play an important role in the development of long-term disorders associated with diabetic complications. In view of the wide occurrence of advanced glycation end products (AGE’s) and the oxidative stress derived from them in a variety of diabetic complications, it would be of great interest to identify and develop AGE inhibitors. In this study, synthesis and in vitro antiglycation activity of a small library of forty urea/thiourea derivatives of Phe/Tyr/Glu/Lys-benzisoxazole hybrids are reported. Structures of the compounds were confirmed by IR, NMR, mass spectrometry, and elemental analysis. Most of the title compounds exhibited promising activity. Best antiglycation activity was found for Tyr analogue with methoxy group as a substituent particularly at the para position with IC50 value of 1.9 μM against the positive control, Rutin, with IC50 = 41.9 μM. Thus, the title compounds represent novel class of potent antiglycating agents.
Similar content being viewed by others
Abbreviations
- AGE’s:
-
advanced glycation end products
- BSA:
-
bovine serum albumin
- EDCI:
-
1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide
- Het:
-
[3-(4-piperidyl)-6-fluoro-1,2-benzisoxazole]
- HOBt:
-
1-hydroxybenzotriazole
- NMM:
-
N-methyl morpholine
- TCA:
-
trichloroacetic acid
References
Rabbani, N. and Thornalley, P.J., Amino Acids, 2012, vol. 42, pp. 1087–1096.
Thorpe, S.R. and Baynes, J.W., Amino Acids, 2003, vol. 25, pp. 275–281.
Ahmed, N., Diabetes. Res. Clin. Pract., 2005, vol. 67, pp. 3–21.
Reddy, V.P. and Beyaz, A., Drug. Discov. Today, 2006, vol. 11, pp. 646–654.
Ramakrishnan, S. and Sulochana, K.N., Exp. Eye. Res., 1993, vol. 57, pp. 623–628.
Sensi, M., De Rossi, M.G., Celi, F.S., Cristina, A., Rosati, C., Prrett, D., Anreani, D., and Di Mario, U., Diabetologia, 1993, vol. 36, pp. 797–801.
Cao, F., Jia, J., Yin, Z., Gao, Y., Sha, L., Lai, Y., Ping, Q., and Zhang, Y., Mol. Pharm., 2012, vol. 9, pp. 2127–2135.
Gupta, D., Gupta, S.V., Lee, K.D., and Amidon, G.L., Mol. Pharm., 2009, vol. 6, p. 1604–1611.
Hughes, R.A. and Moody, C.J., Angew. Chem. Int. Ed., 2007, vol. 46, pp. 7930–7954.
Suresha, G.P., Prakasha, K.C., Shivakumara, K.N., Kapfo, W., and Gowda, D.C., Int. J. Pep. Res. Thera., 2009, vol. 15, pp. 25–30.
Suhas, R., Chandrashekar, S., and Gowda, D.C., Eur. J. Med. Chem., 2011, vol. 46, pp. 704–711.
Suresha, G.P., Suhas, R., Kapfo, W., and Gowda, D.C., Eur. J. Med. Chem., 2011, vol. 46, pp. 2530–2540.
Adams, A.D., Yuen, W., Hu, Z., Santini, C., Jones, A.B., MacNaul, K.L., Berger, J.P., Doebber, T.W., and Moller, D.E., Bioorg. Med. Chem. Lett., 2003, vol. 13, pp. 931–935.
Arakawa, K., Inamasu, M., Matsumoto, M., Okumara, K., Yasuda, K., Akastuka, H., Kawanami, S., Watanabe, A., Homma, K., Saiga, Y., Ozeki, M., and Iijima, I., Chem. Pharm. Bull., 1997, vol. 45, pp. 1984–1993.
Aiello, S., Wells, G., Stone, E.L., Kadri, H., Bazzi, R., Bell, D.R., Stevens, M.F., Matthews, C.S., Bradshaw, T.D., and Westwell, A.D., J. Med. Chem., 2008, vol. 51, pp. 5135–5139.
Suhas, R., Chandrashekar, S., and Gowda, D.C., Eur. J. Med. Chem., 2012, vol. 48, pp. 179–191.
Khan, K.M., Karim, A., Ambreen, N., Saied, S., Rasheed, S., Perveen, S., and Choudhary, M.I., J. Pharm. Res., 2012, vol. 5, pp. 664–665.
Khan, K.M., Saeed, S., Ali, M., Gohar, M., Zahid, J., Khan, A., Perveen, S., and Choudhary, M.I., Bioorg. Med. Chem., 2009, vol. 17, pp. 2447–2451.
Mendez, J.D., Xie, J., and Garcia-Perez, E., WASJ, 2007, vol. 2, pp. 090–098.
Venkatachalam, T.K., Maob, C., and Uckun, F.M., Bioorg. Med. Chem., 2004, vol. 12, pp. 4275–4284.
Tale, R.H., Rodge, A.H., Hatnapure, G.D., Keche, A.P., Patil, K.M., and Pawar, R.P., Med. Chem. Res., 2012, vol. 21, pp. 4252–4260.
Suhas, R., Chandrashekar, S., and Gowda, D.C., Int. J. Pep. Res. Thera., 2012, vol. 18, pp. 89–98.
Suhas, R. and Gowda, D.C., Chem. Biol. Drug. Des., 2012, vol. 79, pp. 850–862.
Perveen, S., Fatima, N., Khan, M.A., Dar, A., Khan, K.M., Afza, N., and Voelter, W., Med. Chem. Res., 2012, vol. 21, pp. 2709–2715.
Rahbar, S. and Figarola, J.L., Arch. Biochem. Biophys., 2003, vol. 419, pp. 63–79.
Strupczewski, J.T., Allen, R.C., Gardner, B.A., Schmid, B.L., Stache, U., Glamkowski, E.J., and Jones, M.C., J. Med. Chem., 1985, vol. 28, pp. 761–769.
Shantharam, C.S., Suyoga Vardhan, D.M., Suhas, R., Sridhara, M.B., and Gowda, D.C., Eur. J. Med. Chem., 2013, vol. 60, pp. 325–332.
Nakagawa, T., Yokozawa, T., Terasawa, K., Shu, S., and Juneja, L.R., J. Agric. Food. Chem., 2002, vol. 50, pp. 2418–2422.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Shantharam, C.S., Suyoga Vardhan, D.M., Suhas, R. et al. Design and synthesis of amino acids-conjugated heterocycle derived ureas/thioureas as potent inhibitors of protein glycation. Russ J Bioorg Chem 40, 443–454 (2014). https://doi.org/10.1134/S1068162014040128
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162014040128