Abstract
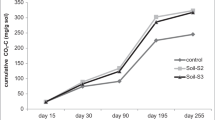
Feeding of the escalating population has subjected today’s agriculture to massive farming that has shattered soil structure, organic carbon and fertility. The vulnerability of applied organics to high temperature make them inappropriate under such circumstances. The help of indigenous exopolysaccharides (EPS) secreting microflora (Pre-isolated (M2, M3, M11, M16, M19 and M22)) was taken in an incubation experiment for the restoration of soil structure and organic carbon. M22 yielded 44.5% aggregation, 8.57 and 6 g kg–1 total and macroaggregate carbon, 220 and 311.2 mg kg–1 dissolved and microbial biomass carbon and 13.143 mg k–1 CO2 evolved in soils having moisture at 100% available water contents (AWC). At the end of the experiment M2, M19 and M22 yielded 43.87, 42.58 and 42.82% (0.25 to >1 mm) water-stable aggregates (WSA) that also carried 5.31, 5.75 and 5.41 g kg–1 carbon, respectively. Microbial activity as CO2 evolved (13.13, 13.73 and 14.11), solution (217.37, 225. 98 and 218.61), biomass carbon (300.93, 287.6 and 303.51) mg kg–1 and total organic carbon (TOC) (8.52, 8.67 and 8.73) g kg–1 were found highest in M2, M19 and M22 treated soils, respectively. Saccharide glues excreted from microbiota, flocculate soil particles to formulate aggregates, entrapping carbon for structural stabilization and organics restoration.







Similar content being viewed by others
REFERENCES
A. Ghani, M. Dexter and K. W. Perrott, “Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation,” Soil Biol. Biochem. 35 (9), 1231–1243 (2003). https://doi.org/10.1016/S0038-0717(03)00186-X
A. J. Franzluebbers, R. L. Haney, C. W. Honeycutt, H. H. Schomberg and F. M. Hons, “Flush of carbon dioxide following rewetting of dried soil relates to active organic pools,” Soil Sci. Soc. Am. 64 (2), 613–623 (2000). https://doi.org/10.2136/sssaj2000.642613x
A. Y. Y. Kong, K. M. Scow, A. L. Córdova-Kreylos, W. E. Holmes and J. Six, “Microbial community composition and carbon cycling within soil microenvironments of conventional, low-input, and organic cropping systems,” Soil Biol. Biochem. 43 (1), 20–30 (2011). https://doi.org/10.1016/j.soilbio.2010.09.005
C. C. Cleveland, D. R. Nemergut, S. K. Schmidt, and A. R. Townsend, “Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition,” Biogeochemistry 82 (3), 229–240 (2007). https://doi.org/10.1007/s10533-006-9065-z
D. Cosentino, C. Chenu, and Y. L. Bissonnais, “Aggregate stability and microbial community dynamics under drying-wetting cycles in a silt loam soil,” Soil Biol. Biochem. 38 (8), 2053–2062 (2006). https://doi.org/10.1016/j.soilbio.2005.12.022
D. S. Jenkinson and D. S. Powlson, “The effects of biocidal treatments on metabolism in soil—I. Fumigation with chloroform,” Soil Biol. Biochem. 8 (3), 209–213 (1976). https://doi.org/10.1016/0038-0717(76)90001-8
E. Armada, G. Portela, A. Roldán, and R. Azcón, “Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions,” Geoderma 232, 640–648 (2014). https://doi.org/10.1016/j.geoderma.2014.06.025
E. Schlichting, H. P. Blume, and K. Stahr, Bodenkundliches Praktikum, Ed. by P. Parey (Hamburg, 1995).
H. Bossuyt, K. Denef, J. Six, S. D. Frey, R. Merckx, and K. Paustian, “Influence of microbial populations and residue quality on aggregate stability,” App. Soil Ecol. 16 (3), 195–208 (2001). https://doi.org/10.1016/S0929-1393(00)00116-5
H. Shahzad, M. Iqbal, and Q. U. Khan, “Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria,” Turk. J. Biochem. 43 (6), 686–692 (2018). https://doi.org/10.1515/tjb-2017-0204
H. C. Flemming and J. Wingender, “The biofilm matrix,” Nat. Rev. 8 (9), 623–633 (2010). https://doi.org/10.1038/nrmicro2415
H. M. Alvarez, R. A. Silva, A. C. Cesari, A. L. Zamit, S. R. Peressutti, R. Reichelt, U. Keller, U. Malkus, C. Rasch, and T. Maskow, “Physiological and morphological responses of the soil bacterium Rhodococcus opacusstrain PD630 to water stress,” FEMS Microbiol. Ecol. 50 (2), 75–86 (2004). https://doi.org/10.1016/j.femsec.2004.06.002
J. Six and K. Paustian, “Citation classic XII: Aggregate-associated soil organic matter as an ecosystem property and measurement tool,” Soil Biol. Biochem. 68, A4–A9 (2014). https://doi.org/10.1016/j.soilbio.2013.06.014
J. Six, E. T. Elliott, and K. Paustian, “Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture,” Soil Biol. Biochem. 32 (14), 2099–2103 (2000). https://doi.org/10.1016/S0038-0717(00)00179-6
J. Six, E. T. Elliott, K. Paustian, and J. W. Doran, “Aggregation and soil organic matter accumulation in cultivated and native grassland soils,” Soil Sci. Soc. Am. J. 62 (5), 1367–1377 (1998). https://doi.org/10.2136/sssaj1998.03615995006200050032x
J. Six, H. Bossuyt, S. Degryze, and K. Denef, “A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics,” Soil Tillage Res. 79 (1), 7–31 (2004). https://doi.org/10.1016/j.still.2004.03.008
J. Six, S. D. Frey, R. K. Thiet, and K. M. Batten, “Bacterial and fungal contributions to carbon sequestration in agroecosystems,” Soil Sci. Soc. Am. J. 70 (2), 555–569 (2006). https://doi.org/10.2136/sssaj2004.0347
J. Williams, R. E. Prebble, W. T. Williams, and C. T. Hignett, “The influence of texture, structure and clay mineralogy on the soil moisture characteristic,” Aust. J. Soil Res. 20, 15–32 (1983).
J. G. Caporaso, C. L. Lauber, W. A. Walters, D. Berg-Lyons, C. A. Lozupone, P. J. Turnbaugh, N. Fierer and R. Knight, “Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample,” Proc. Natl. Acad. Sci. U.S.A. 108, 4516–4522 (2011). https://doi.org/10.1073/pnas.1000080107
J. H. Campbell, J. S. Clark, and J. C. Zak, “PCR-DGGE comparison of bacterial community structure in fresh and archived soils sampled along a Chihuahuan Desert elevational gradient,” Microb. Ecol. 57 (2), 261–266 (2009). https://doi.org/10.1007/s00248-008-9479-3
J. H. Dane and J. W. Hopmans, “Laboratory determination of water retention,” in Methods of Soil Analysis, Part 4: Physical Methods, Ed. by J. H. Danea and G. C. Topp (Soil Science Society of America, Madison, WI, 2002), pp. 671–720.
J. H. Priester, Y. Ge, V. Chang, P. K. Stoimenov, J. P. Schimel, G. D. Stucky, and P. A. Holden, “Assessing interactions of hydrophilic nanoscale TiO2 with soil water,” J. Nanopart. Res. 15, 1899 (2013). https://doi.org/10.1007/s11051-013-1899-4
J. M. Tisdall, “Possible role of soil microorganisms in aggregation in soils,” Plant Soil 159 (1), 115–121 (1994). https://doi.org/10.1007/BF00000100
J. P. Schimel and S. M. Schaeffer, “Microbial control over carbon cycling in soil,” Front. Microbiol. 3, 348 (2012). https://doi.org/10.3389/fmicb.2012.00348
J. S. Kim, G. Sparovek, R. M. Longo, W. J. De-Melo, and D. Crowley, “Bacterial diversity of terra preta and pristine forest soil from the Western Amazon,” Soil Biol. Biochem. 39 (2), 684–690 (2007). https://doi.org/10.1016/j.soilbio.2006.08.010
L. F. Roesch, R. R. Fulthorpe, A. Riva, G. Casella, A. K. Hadwin, A. D. Kent, S. H. Daroub, F. A. Camargo, W. G. Farmerie, and E. W. Triplett, “Pyrosequencing enumerates and contrasts soil microbial diversity,” ISME J. 1 (4), 283–290 (2007).
L. M. Zibilski, “Carbon mineralization,” in Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties, SSSA Book Series no. 5, Ed. by J. M. Bingham and S. H. Mickelson (Soil Science Society of America, Madison, WI, 1994), pp. 853–863.
M. Clarholm and U. Skyllberg, “Translocation of metals by trees and fungi regulates pH, soil organic matter turnover and nitrogen availability in acidic forest soils,” Soil Biol. Biochem. 63, 142–153 (2013). https://doi.org/10.1016/j.soilbio.2013.03.019
M. Clarholm, U. Skyllberg, and A. Rosling, “Organic acid induced release of nutrients from metal-stabilized soil organic matter—the unbutton model,” Soil Biol. Biochem. 84, 168–176 (2015). https://doi.org/10.1016/j.soilbio.2015.02.019
M. Kleber, K. Eusterhues, M. Keiluweit, C. Mikutta, R. Mikutta, and P. S. Nico, “Mineral–organic associations: formation, properties, and relevance in soil environments,” Adv. Agron. 130, 1–140 (2015). https://doi.org/10.1016/bs.agron.2014.10.005
M. A. Redmile-Gordon, P. C. Brookes, R. P. Evershed, K. W. T. Goulding and P. R. Hirsch, “Measuring the soil-microbial interface: extraction of extracellular polymeric substances (EPS) from soil biofilms,” Soil Biol. Biochem. 72, 163–171 (2014). https://doi.org/10.1016/j.soilbio.2014.01.025
M. V. Cheshire, “Origins and stability of soil polysaccharide,” J. Soil Sci. 28 (1), 1–10 (1977). https://doi.org/10.1111/j.1365-2389.1977.tb02290.x
N. Fierer and R. B. Jackson, “The diversity and biogeography of soil bacterial communities,” Proc. Natl. Acad. Sci. U.S.A. 103, 626–631 (2006). https://doi.org/10.1073/pnas.0507535103
P. Dlamini, P. Chivenge, A. Manson, and V. Chaplot, “Land degradation impact on soil organic carbon and nitrogen stocks of sub-tropical humid grasslands in South Africa,” Geoderma 235, 372–381 (2014). https://doi.org/10.1016/j.geoderma.2014.07.016
P. Lavelle, D. Bignell, M. Lepage, V. Wolters, P. Roger, P. Ineson, O. W. Heal, and S. Dhillion, “Soil function in a changing world: the role of invertebrate ecosystem engineers,” Eur. J. Soil Biol. 33, 159–193 (1997).
P. A. Holden, “How do the microhabitats framed by soil structure impact soil bacteria and the processes they catalyze?” in The Architecture and Biology of Soils: Life in Inner Space, Ed. by K. Ritz and I. Young (CAB International, Wallingford, 2011), pp. 1–62. https://doi.org/10.1038/nrmicro2415
R. Dudal, “The sixth factor of soil formation,” Eurasian Soil Sci. 38, 12–21 (2005).
R. K. Monson, D. L. Lipson, S. P. Burns, A. A. Turnipseed, A. C. Delany, M. W. Williams, and S. K. Schmidt, “Winter forest soil respiration controlled by climate and microbial community composition,” Nature 439 (7077), 711–714 (2006). .https://doi.org/10.1038/nature04555
S. Abiven, S. Menasseri, and C. Chenu, “The effects of organic inputs over time on soil aggregate stability—a literature analysis,” Soil Biol. Biochem. 41, 1–12 (2009). https://doi.org/10.1016/j.soilbio.2008.09.015
S. Degryze, J. Six, C. Brits, and R. Merckx, “A quantification of short-term macroaggregate dynamics: influences of wheat residue input and texture,” Soil Biol. Biochem. 37 (1), 55–66 (2005). https://doi.org/10.1016/j.soilbio.2004.07.024
S. Manzoni, J. P. Schimel, and A. Porporato, “Responses of soil microbial communities to water stress: results from meta-analysis,” Ecology 93 (4), 930–938 (2012). https://doi.org/10.1890/11-0026.1
S. S. Briar, S. J. Fonte, I. Park, J. Six, K. Scow, and H. Ferris, “The distribution of nematodes and soil microbial communities across soil aggregate fractions and farm management systems,” Soil. Biol. Biochem. 43 (5), 905–914 (2011). https://doi.org/10.1016/j.soilbio.2010.12.017
T. An, S. Schaeffer, J. Zhuang, M. Radosevich, S. Li, H. Li, J. Pei, and J. Wang, “Dynamics and distribution of 13C-labeled straw carbon by microorganisms as affected by soil fertility levels in the Black Soil region of Northeast China,” Biol. Fertil. Soils 51 (5), 605–613 (2015). https://doi.org/10.1007/s00374-015-1006-3
X. Chen, Z. Li, M. Liu, C. Jiang, and Y. Che, “Microbial community and functional diversity associated with different aggregate fractions of a paddy soil fertilized with organic manure and/or NPK fertilizer for 20 years,” J. Soils Sediments 15 (2), 292–301 (2015). https://doi.org/10.1007/s11368-014-0981-6
X. Peng, Q. Zhu, Z. Zhang, and P.D. Hallet, “Combined turnover of carbon and soil aggregates using rare earth oxides and isotopically labelled carbon as tracers,” Soil Biol. Biochem. 109, 81–94 (2017). https://doi.org/10.1016/j.soilbio.2017.02.002
X. Peng, R. Horn, and P. Hallett, “Soil structure and its functions in ecosystems: phase matter and scale matter,” Soil Tillage Res. 146, 1–3 (2015). https://doi.org/10.1016/j.still.2014.10.017
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haroon Shahzad Rhizobacterial Inoculation to Quantify Structural Stability and Carbon Distribution in Aggregates of Sandy Clay Loam Soil. Eurasian Soil Sc. 53, 675–685 (2020). https://doi.org/10.1134/S1064229320050142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229320050142