Abstract
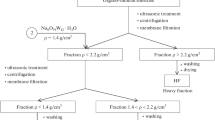
The mineralization and humification dynamics of corn plant residues in loamy and sandy substrates have been studied under laboratory conditions. It has been shown that the dynamics are determined by the undulating development laws of the microbial community under constant temperature and moisture conditions. At the same time, the intensity and final results of the processes significantly differ depending on the composition and properties of the mineral substrate. The loss of Corg during the mineralization and the content of newly formed humic substances reached the maximum values a month after the beginning of the experiment. The mineralization is more intensive in sand at the early stages, and the humification is more active in loam throughout the incubation period. The loamy substrate has better protective properties compared to the sand; therefore, it favors the accumulation of significant amounts of fulvic acids (FAs), along with humic acids (HAs), and causes the relative fulvatization of the humic substances. It has been found using densimetric fractionation and Fourier IR spectroscopy that the different mineralogy of the fractions results in differences in the chemical composition of the formed mineral-organic compounds of newly formed humic substances, mainly due to carboxyl and nitrogen-containing groups. The similarity of the humification products in the heavy fractions of the loamy and sandy substrates has been revealed.
Similar content being viewed by others
References
L. N. Aleksandrova, Soil Organic Matter and the Processes of Its Transformation, (Nauka, Leningrad, 1980) [in Russian].
L. H. Aleksandrova, “Humus-forming processes in soil,” in Soil Humus Substances, Zap. Leningr. Sel’skokhoz. Inst., 142, 26–82 (1970).
L. N. Aleksandrova and V. F. Arshavskaya, “Changes in the composition of humic acids in the course of humification of plant residues,” Zap. Leningr. Sel’skokhoz. Inst., 117, 14–21 (1966).
T. V. Alekseeva, P. B. Kabanov, B. N. Zolotareva, A. O. Alekseev, V. A. Alekseeva, “Humic substances in the palygorskite organomineral complex from fossil soil of the Late Carboniferous period in the southern Moscow region,” Dokl. Akad. Nauk. 425(2), 265–270 (2009).
N. N. Bambalov, A. V. Khoruzhik, and N. S. Yankovskaya, “Regularities and specific features of humification in peat soils,” in Organic Matter of Soils and Methods of Its Study (LSKhI, Leningrad, 1990), pp. 29–33 [in Russian].
L. J. Bellamy, The Infrared Spectra of Complex Molecules, 2nd ed. (Methuen, London, 1959).
A. Ya. Vanyushina and L. S. Travnikova, “Organic-Mineral Interactions in Soils: A Review,” Eur. Soil Sci. 36(4), 379–387 (2003).
Humic Substances in the Biosphere, Ed. by D. S. Orlov (Nauka, Moscow, 1993) [in Russian].
T. A. Zubkova and L. O. Karpachevskii, Matrix Organization of Soils (Rusaki, Moscow, 2001) [in Russian].
M. M. Kononova, Soil Organic Matter (Izd. Akad. Nauk SSSR, Moscow, 1963) [in Russian].
M. M. Kononova, Problems and Challenges of Studying Soil Humus (Izd. Akad. Nuak SSSR, Moscow, 1951) [in Russian].
G. N. Kurochkina and D. L. Pinskii, “Kinetics and thermodynamics of polyelectrolyte adsorption on synthetic aluminosilicate gels,” Eur. Soil Sci. 36(2), 155–163 (2003).
G. N. Kurochkina and D. L. Pinskii, “The formation of mineral-organic compounds and their effect on the surface properties of soil aluminosilicates,” Eur. Soil Sci. 37(4), 378–387 (2004).
M. F. Lyuzhin, “Mineralization and humification of plant residues in soil,” Zap. Leningr. Sel’skokhoz. Inst. 117(1), 27–39 (1968).
E. G. Morgun and M. I. Makarov, “Use of sodium polytungstate in the granulo-densimetric fractionation of soil material,” Eur. Soil Sci. 44(4), 394–398 (2011).
A. M. Semenov, V. M. Semenov, and A. van Bruggen, “Diagnosis of the health and quality of soils,” Agrokhimiya, No. 12, 4–20 (2011).
D. S. Orlov, Humic Acids of Soils and a General Theory of Humification (Izd. Mosk. Gos. Univ., Moscow, 1990) [in Russian].
D. S. Orlov, “Organic substances of Russian soils,” Eur. Soil Sci. 31(9), 946–953 (1998).
D. S. Orlov and L. A. Grishina, Practicum on Humus Chemistry (Izd. Mosk. Gos. Univ., Moscow, 1981) [in Russian].
D. S. Orlov and N. N. Osipova, Infrared Spectra of Soils and Soil Components (Izd. Mosk. Gos. Univ., Moscow, 1988) [in Russian].
D. L. Pinskii, Ion-Exchange Processes in Soils (ONTI, Pushchino, 1997) [in Russian].
D. L. Pinskii and G. N. Kurochkina, “Evolution of theories on soil adsorption capacity,” in Soil Processes and Spatio-Temporal Organization of Soils (Nauka, Moscow, 2006), pp. 295–312 [in Russian].
A. V. Rybalkina and E. V. Kononenko, “Microflora of decomposing plant residues,” Pochvovedenie, No. 5, 21–34 (1959).
L. S. Travnikova, “Patterns of humus accumulation: new data and their interpretation,” Eur. Soil Sci. 35(7), 737–748 (2002).
L. S. Travnikova, N. A. Titova, and M. Sh. Shaimukhametov, “The role of products of interaction between organic and mineral componensts in the genesis and fertility of soils,” Pochvovedenie, No. 10, 81–97 (1992).
V. A. Kholodov, A. I. Konstantinov, and I. V. Perminova, “The carbon distribution among the functional groups of humic acids isolated by sequential alkaline extraction from gray forest soil,” Eur. Soil Sci. 42(11), 1229–1233 (2009).
O. G. Chertov, A. S. Komarov, and M. A. Nadporozhskaya, “Analysis of the dynamics of plant residue mineralization and humification in soil,” Eur. Soil Sci. 40(2), 140–148 (2007).
M. Sh. Shaimukhametov, N. A. Titova, L. S. Travni- kova, and E. M. Lebenets, “Application of physical fractionation methods to characterize soil organic matter,” Pochvovedenie, No. 8, 131–141 (1984).
U. Birkel, G. Gerold, and J. Niemeyer, “Abiotic reactions of organics on clay mineral surfaces,” Developments in Soil Science. 28Part 1, 437–447 (2002).
C. Chenu and A. F. Plante, “Clay-sized organo-mineral complexes in a cultivation chronosequence: revisiting the concept of the “primary organo-mineral complex”, Eur. J. Soil Sci. 57(4), 596–607 (2006).
D. P. Dick, J. H. Z. Santos, and E. M. Ferranti, “Chemical characterization and infrared spectroscopy of soil organic matter from two southern Brazilian soils,” R. Bras. Ci. Solo. 27(1), 29–39 (2003).
G. W. Ford and D. J. Creenland, “The dynamics of partially humified organic matter in some arable soils,” Trans. 9th Intern. Congr. Soil Sci., Adelaide, Australia, 1968, pp. 403–410.
W. Flaig, “Organic compounds in soil,” Soil Sci. 111(1), 19–33 (1971).
K. Kaiser and G. Guggenberger, “Mineral surfaces and soil organic matter,” Eur. J. Soil Sci. 54(Iss. 2), 219–236 (2003).
K. Kaiser, G. Guggenberger, L. Haumaier, and W. Zech, “Dissolved organic matter sorption on sub soils and minerals studied by 13C-NMR and DRIFT spectroscopy,” Eur. J. Soil Sci. 48(2), 301–310 (1997).
J. Lehmann, J. Kinyangi, and D. Solomon, “Organic matter stabilization in soil microaggregates: implications from spatial heterogeneity of organic carbon contents and carbon forms,” Biogeochemistry. 85(1), 45–57 (2007).
J. Magid, A. Gorissen, and K. E. Giller, “In search of the elusive “active” fraction of soil organic matter: three size-density fractionation methods for tracing the fate of homogeneously 14C-labelled plant material,” Soil Biol. Biochem. 28(1), 89–99 (1996).
R. Mikutta, M. Kleber, M. S. Torn, and R. Jahn, “Stabilization of soil organic matter: association with minerals or chemical recalcitrance?,” Biogeochemistry. 77(1), 25–56 (2006).
M. Schnitzer, “Soil organic matter,” Proc. 3rd Intern. Symp., Vienna (Intern. At. En. Agency, 1977), pp. 117–132.
J. Six, E. T. Elliot, K. Paustian, and J. W. Doran, “Aggregation and soil organic matter accumulation in cultivated and native grassland soils,” Soil Sci. Soc. Am. J. 62(5), 1367–1377 (1998).
J. Six, P. Callewaert, S. Lenders, S. De Gryze, S. J. Morris, E. G. Gregorich, E. A. Paul, K. Paustian, “Measuring and understanding carbon storage in afforested soils by physical fractionation,” Soil Sci. Soc. Am. J. 66(6), 1981–1987 (2002).
P. Sollins, M. G. Kramer, C. Swanston, K. Lajtha, T. Filley, A. K. Aufdenkampe, R. Wagai, R. D. Bowden, “Sequential density fractionation across soils of contrasting mineralogy: evidence for both microbial- and mineral-controlled soil organic matter stabilization,” Biogeochemistry. 96(1–3), 209–231 (2009).
P. Sollins, C. Swanston, M. Kleber, T. Filley, M. Kramer, S. Crow, B. A. Caldwell, K. Lajtha, and R. Bowden, “Organic C and N stabilization in a forest soil: evidence from sequential density fractionation,” Soil Biol. Biochem. 38(11), 3313–3324 (2006).
L. W. Turchenek and J. M. Oades, “Fractionation of organo-mineral complexes by sedimentation and density techniques,” Geoderma. 21 (1979).
M. Van Lützow, I. Kögel-Knabner, K. Ekschmitt, E. Matzner, G. Guggenberger, B. Marschner, H. Flessa, “Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review,” Eur. J. Soil Sci. 57(4), 426–445 (2006).
A. G. Zavarzina, “A mineral support and biotic catalyst are essential in the formation of highly polymeric soil humic substances,” Eur. J. Soil Sci. 39(Suppl. 1), 48–53 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.N. Mal’tseva, B.N. Zolotareva, D.L. Pinskii, 2013, published in Pochvovedenie, 2013, No. 10, pp. 1239–1252.
Rights and permissions
About this article
Cite this article
Mal’tseva, A.N., Zolotareva, B.N. & Pinskii, D.L. Transformation of corn plant residues in loamy and sandy substrates. Eurasian Soil Sc. 47, 466–477 (2014). https://doi.org/10.1134/S1064229314050147
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229314050147