Abstract
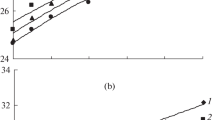
The temperature dependences of the veloсity of hypersound (V(T)) in solutions with various concentrations of guanidine hydrochloride (GndHCl) are studied by Brillouin–Mandelstam light scattering spectroscopy in the temperature range from 263 to 353 K. It is shown that the temperature dependence of the sound velocity has a pronounced maximum at low concentrations of GndHCl. An increase in the concentration of GndHCl in a solution is accompanied by a shift of the maximum to the low-temperature region and an increase in the absolute values of the sound velocity, which means a decrease in the adiabatic compressibility over the entire range of studied temperatures. The temperature dependence of the adiabatic compressibility is constructed at low concentrations of GndHCl. The contribution of relaxation processes to the temperature behavior of the attenuation of hypersound in solutions of GndHCl is determined. It is shown that their behavior is activated thermally and described by the Arrhenius law. The values of the activation energy of relaxation processes are calculated. Possible mechanisms that underlie the observed phenomena are discussed.





Similar content being viewed by others
REFERENCES
E. D. Raczynska, M. K. Cyranski, M. Gutowski, J. Rak, J.-F. Gal, P.-C. Maria, M. Darowska, and K. Duczmal, J. Phys. Org. Chem. 16 (2), 91 (2003). https://doi.org/10.1002/poc.578
P. J. Bailey and S. Pace, Coord. Chem. Rev. 214 (1), 91 (2001). https://doi.org/10.1016/S0010-8545(00)00389-1
R. G. S. Berlinck, Nat. Prod. Rep. 16, 339 (1999). https://doi.org/10.1039/A900338J
K. Gopi, B. Rathi, and N. Thirupathi, J. Chem. Sci. 122 (2), 157 (2010). https://doi.org/10.1007/s12039-010-0017-8
T. Yamada, X. Liu, U. Englert, H. Yamane, and R. Dronskowski, Chem. Eur. J. 15 (23), 5651 (2009). https://doi.org/10.1002/chem.200900508
M. Y. Schrier and E. E. Schrier, J. Chem. Eng. Data 22 (1), 73 (1977). https://doi.org/10.1021/je60072a009
K. Kawahara and C. Tanford, J. Biol. Chem. 241 (13), 3228 (1966).
F. Hofmeister, Arch. Exp. Pathol. Pharmakol. 24, 247 (1888).
S. Moelbert, B. Normand, and P. D. L. Rios, Biophys. Chem. 112 (1), 45 (2004). https://doi.org/10.1016/j.bpc.2004.06.012
Y. Zhang and P. S. Cremer, Curr. Opin. Chem. Biol. 10 (6), 658 (2006). https://doi.org/10.1016/j.cbpa.2006.09.020
P. E. Mason, G. W. Neilson, C. E. Dempsey, A. C. Barnes, and J. M. Cruickshank, PNAS 100 (8), 4557 (2003). https://doi.org/10.1073/pnas.0735920100
E. P. O’Brien, R. I. Dima, B. Brooks, and D. Thirumalai, J. Am. Chem. Soc. 129 (23), 7346 (2007). https://doi.org/10.1021/ja069232
P. E. Mason, G. W. Neilson, J. E. Enderby, M.-L. Saboungi, and C. E. Dempsey, J. Am. Chem. Soc. 126 (37), 11462 (2004). https://doi.org/10.1021/ja040034x
L. S. Hibbard and A. Tulinsky, Biochemistry 17 (25), 5460 (1978). https://doi.org/10.1021/bi00618a021
S. C. Mande and M. E. Sobhia, Protein Eng. 13 (2), 133 (2000). https://doi.org/10.1093/protein/13.2.133
J. Dunbar, H. P. Yennawar, S. Banerjee, J. Luo, and G. K. Farber, Protein Sci. 6 (8), 1727 (1997). https://doi.org/10.1002/pro.5560060813
A. Möglich, F. Krieger, and T. Kiefhaber, J. Mol. Biol. 345 (1), 153 (2005). https://doi.org/10.1016/j.jmb.2004.10.036
O. Bieri, J. Wirz, B. Hellrung, M. Drewello, and T. Kiefhaber, PNAS 96 (17), 9597 (1999). https://doi.org/10.1073/pnas.96.17.9597
O. Conde, J. Teixeira, and P. Papon, J. Chem. Phys. 76 (7), 3747 (1982). https://doi.org/10.1063/1.443413
A. V. Svanidze, S. G. Lushnikov, and S. Kojima, JETP Lett. 90 (1), 80 (2009). https://doi.org/10.1134/S0021364009130165
D. W. James and R. Appleby, J. Phys. Chem. 86 (14), 2788 (1982). https://doi.org/10.1021/j100211a045
M. E. Gallina, L. Comez, S. Perticaroli, A. Morresi, A. Cesàro, O. De Giacomo, S. Di Fonzo, A. Gessini, C. Masciovecchio, L. Palmieri, M. Paolantoni, P. Sassi, and F. Scarponi, Philos. Mag. 88 (33–35), 3991 (2008). https://doi.org/10.1080/14786430802481903
D. R. Lide, CRC Handbook of Chemistry and Physics, 70th ed. (CRC Press, Boca Raton, FL, 1990).
S. Boudon, G. Wipff, and B. Maigret, J. Phys. Chem. 94 (15), 6056 (1990). https://doi.org/10.1021/j100378a078
F. Bencivenga, A. Cunsolo, M. Krisch, G. Monaco, L. Orsingher, G. Ruocco, F. Sette, and A. Vispa, Phys. Rev. Lett. 98 (8–23), 085501 (2007). https://doi.org/10.1103/PhysRevLett.98.085501
Haruki Takayama, Tomohiko Shibata, Yuto Kuroda, Shota Koda, and Seiji Kojima, Jpn. J. Appl. Phys. 53 (7S), 07KB06 (2014). https://doi.org/10.7567/JJAP.53.07KB06
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Kadkin
Rights and permissions
About this article
Cite this article
Fedoseev, A.I., Koludarov, I.P., Fronttsek, A.V. et al. Elastic Properties of Guanidine Hydrochloride Solutions with Various Concentrations in the Gigahertz Frequency Range. Tech. Phys. 65, 1484–1490 (2020). https://doi.org/10.1134/S1063784220090170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063784220090170