Abstract
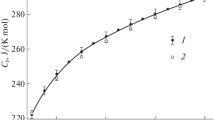
The temperature dependence of the molar heat capacity of HoMnO3 has been measured by differential scanning calorimetry. The experimental data have been used to calculate the thermodynamic properties of the oxide compound (changes in the enthalpy H°(T)–H°(364 K), entropy S°(T)–S°(364 K), and reduced Gibbs energy Φ°(T)). The data on the heat capacity of HoMnO3 have been generalized in the range of 40–1000 K.
Similar content being viewed by others
References
V. E. Wood, A. E. Austin, E. W. Colings, and K. C. Brog, J. Phys. Chem. 34, 859 (1973).
H. W. Brinks, H. Fjellvåg, and A. Kjekshus, J. Solid State Chem. 129, 334 (1997).
A. N. Grundy, M. Chen, B. Hallstedt, and L. J. Gauckler, J. Phase Equilib. Diffus. 26 (2), 131 (2005).
S. Remsen and B. Dabrowski, Chem. Mater. 23, 3818 (2011).
C. N. R. Rao, A. Sundaresan, and R. Saha, J. Phys. Chem. Lett. 3, 2237 (2012).
S. M. Selbach, A. N. Lovik, K. Bergum, J. R. Tolchard, and M.-A. Einarsud, J. Solid State Chem. 196, 528 (2012).
D. G. Tomuta, S. Ramakrishnan, G. J. Neiwenkuys, and J. A. Mydosh, J. Phys.: Condens. Matter 13, 4543 (2001).
C. Fan, Z. Y. Zhao, J. D. Song, J. C. Wu, F. B. Zhang, and X. F. Sun, J. Cryst. Growth 388, 54 (2014).
E. Pawlas-Foryst, K. T. Jacob, and K. Fitzner, Arch. Metall. Mater. 51 (2), 253 (2006).
L. Ghivelder, I. A. Castilo, M. A. Gusma[tilde]o, J. A. Alonso, and L. F. Cohen, Phys. Rev. B: Condens. Matter 60, 12184 (1999).
H. Saton, M. Takagi, K.-I. Kinukawa, and N. Kamegashira, Thermochim. Acta 299, 123 (1997).
H. Saton, J.-I. Iwasaki, K. Kawase, and N. Kamegashira, J. Alloys Compd. 268, 42 (1998).
B. Lorenz, F. Yen, M. M. Gospodinov, and C. W. Chu, Phys. Rev. B: Condens. Matter 71, 014438 (2005).
P. Liu, X.-L. Wang, Z.-X. Chen, Y. Du, and H. Kimura, Phys. Rev. B: Condens. Matter 83, 144404 (2011).
A. Midya, S. N. Das, P. Mandal, S. Pandya, and V. Ganesan, Phys. Rev. B: Condens. Matter 84, 235127 (2011).
L. A. Solovyov, J. Appl. Crystallogr. 37, 743 (2004).
L. T. Denisova, L. G. Chumilina, N. V. Belousova, and V. M. Denisov, Russ. J. Phys. Chem. A 89 (8), 1335 (2015).
V. M. Denisov, L. T. Denisova, L. A. Irtyugo, and V. S. Biront, Phys. Solid State 52 (7), 1362 (2010).
A. G. Gamzatov, A. M. Aliev, K. Sh. Khizriev, I. K. Kamilov, A. S. Mankevich, and I. E. Korsakov, Phys. Solid State 53 (11), 2271 (2011).
V. M. Denisov, L. T. Denisova, L. A. Irtyugo, G. S. Patrin, N. V. Volkov, and L. G. Chumilina, Phys. Solid State 54 (11), 2205 (2012).
N. N. Loshkareva, A. S. Moskvin, and A. M. Balbashov, Phys. Solid State 51 (5), 930 (2009).
S. V. Vonsovskii, Magnetism (Nauka, Moscow, 1971; Wiley, New York, 1974).
C. G. Maier and K. K. Kelley, J. Am. Chem. Soc. 54 (8), 3243 (1932).
P. Richet and G. Fiquet, J. Geophys. Res., B 96 (1), 445 (1991).
H. Satoh, T. Shoji, J. Iwasaki, and N. Kamegashira, Thermochim. Acta 261, 47 (1995).
J. Leitner, P. Chuchvalec, D. Sedmidubský, A. Strejc, and P. Abrman, Thermochim. Acta 395, 27 (2003).
L. T. Denisova, Yu. F. Kargin, and V. M. Denisov, Phys. Solid State 57 (8), 1699 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.T. Denisova, L.G. Chumilina, K.A. Shaikhutdinov, G.S. Patrin, V.M. Denisov, 2016, published in Fizika Tverdogo Tela, 2016, Vol. 58, No. 3, pp. 469–472.
Rights and permissions
About this article
Cite this article
Denisova, L.T., Chumilina, L.G., Shaikhutdinov, K.A. et al. Heat capacity and thermodynamic properties of HoMnO3 in the range of 364–1046 K. Phys. Solid State 58, 481–484 (2016). https://doi.org/10.1134/S1063783416030070
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783416030070