Abstract
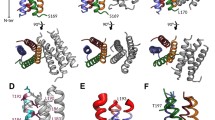
A complex structural analysis of nuclear export protein NS2 (NEP) of influenza virus A has been performed using bioinformatics predictive methods and small-angle X-ray scattering data. The behavior of NEP molecules in a solution (their aggregation, oligomerization, and dissociation, depending on the buffer composition) has been investigated. It was shown that stable associates are formed even in a conventional aqueous salt solution at physiological рН value. For the first time we have managed to get NEP dimers in solution, to analyze their structure, and to compare the models obtained using the method of the molecular tectonics with the spatial protein structure predicted by us using the bioinformatics methods. The results of the study provide a new insight into the structural features of nuclear export protein NS2 (NEP) of the influenza virus A, which is very important for viral infection development.
Similar content being viewed by others
References
N. H. Acheson, Fundamentals of Molecular Virology (Wiley, New York, 2006).
K. Das, J. Aramini, and L-C. Ma, Nat. Struct. Mol. Biol. 17, 530 (2010).
B. Mänz, M. Schwemmle, and L. Brunotte, J. Virol. 87, 7200 (2013).
Y. Zhang, BMC Bioinformatics 9, 40 (2008).
V. Darapaneni, V. K. Prabhaker, and A. Kukol, J. Gen. Virol. 90, 2124 (2009).
H. Akarsu, W. Burmeister, and C. Petosa, EMBO J. 22, 4646 (2003).
D. Paterson and E. Fodor, PLoS Pathog. 8, 1 (2012).
B. Mänz, L. Brunotte, P. Reuther, et al., Nat. Commun. 3, 802 (2012).
P. Reuther, S. Giese, and V. Götz, J. Virol. 88, 263 (2014).
E. C. Hutchinson, E. M. Denham, and B. Thomas, PLoS Pathog. 8, 1 (2012).
T. Gorai, H. Goto, and T. Noda, Proc. Natl. Acad. Sci. USA 109, 4615 (2012).
S. Huang, J. Chen, and Q. Chen, J. Virol. 87, 767 (2013).
J. Yasuda, S. Nakada, A. Kato, et al., Virol. J. 196, 249 (1993).
S. Boulo, H. Akarsu, R. Ruigrok, et al., Virus Res. J. 124, 12 (2007).
L. Brunotte, J. Flies, and H. Bolte, J. Biol Chem. 289, 20067 (2014).
T. Shimizu, N. Takizawa, and K. Watanabe, FEBS Lett. 585, 41 (2011).
K. Watanabe, T. Shimizu, S. Noda, et al., FEBS Open Bio. 4, 683 (2014).
A. O. Golovko, O. N. Koroleva, and V. L. Drutsa, Proc. XXIX Winter School of Young Scientists “Promising Directions in Physicochemical Biology and Biotechnology”, Moscow, February 7–10, 2017.
U. K. Laemmli, Nature 227, 680 (1970).
A. L. Ksenofontov, V. S. Kozlovskii, and L. V. Kordyukova, Mol. Biol. 40, 172 (2006).
G. P. S. Raghava, Proc. Conf. CASP5, California, December 1–5, 2000, p. 132.
A. Drozdetskiy, C. Cole, J. Procter, et al., Nucl. Acids Res. 43, 389 (2015).
J. Cuff and G. J. Barton, Proteins 40, 502 (1999).
D. W. A. Buchan, F. Minneci, T. C. O. Nugent, et al., Nucl. Acids Res. 41, 340 (2013).
R. Adamczak, A. Porollo, and J. Meller, Proteins: Struct., Funct., Bioinf. 56, 753 (2004).
R. Adamczak, A. Porollo, and J. Meller, Proteins: Struct., Funct., Bioinf. 59, 467 (2005).
M. Wagner, R. Adamczak, A. Porollo, et al., J. Comput. Biol. 12, 355 (2005).
A. Porollo, R. Adamczak, M. Wagner, et al., Proc. Conf. CIRAS, Singapore, December 15–18, 2003, p. 1.
M. Källberg, H. Wang, S. Wang, et al., Nat. Protoc. 7, 1511 (2012).
L. A. Kelley, S. Mezulis, C. M. Yates, et al., Nat. Protoc. 10, 845 (2015).
J. Haas, S. Roth, K. Arnold, et al., Database (Oxford), Bat031, 1 (2013).
M. Kallerg, H. Wang, J. Peng, et al., Nat. Protoc. 7, 1511 (2012).
Z. Dosztányi, V. Csizmók, P. Tompa, et al., J. Mol. Biol. 347, 827 (2005).
Z. Dosztányi, V. Csizmók, P. Tompa, et al., Bioinformatics 21, 3433 (2005).
R. Linding, L. J. Jensen, F. Diella, et al., Structure 11, 453 (2003).
O. V. Galzitskaya, S. O. Garbuzynskiy, and M. Yu. Lobanov, Bioinformatics 22, 2948 (2006).
O. V. Galzitskaya, S. O. Garbuzynskiy, and M. Yu. Lobanov, Mol. Biol. 40, 341 (2006).
O. V. Galzitskaya, S. O. Garbuzynskiy, and M. Yu. Lobanov, PLoS Comput. Biol. 2, e177 (2006).
O. V. Galzitskaya, S. O. Garbuzynskiy, and M. Yu. Lobanov, Protein Sci. 13, 2871 (2004).
L. J. McGuffin, Bioinformatics 24, 1798 (2008).
C. E. Blanchet, A. Spilotros, F. Schwemmer, et al., J. Appl. Crystallogr. 48, 431 (2015).
P. V. Konarev, V. V. Volkov, A. V. Sokolova, et al., Appl. Crystallogr. 36, 1277 (2003).
A. Guinier, Ann. Phys. 12, 161 (1939).
G. Porod, Small-Angle X-Ray Scattering (Academic, London, 1982), p. 17.
C. E. Blanchet and D. I. Svergun, Annu. Rev. Phys. Chem. 64, 37 (2013).
D. I. Svergun, J. Appl. Crystallogr. 25, 495 (1992).
D. I. Svergun, Biophys. J. 76 (6), 2879 (1999).
V. V. Volkov and D. I. Svergun, J. Appl. Crystallogr. 36, 860 (2003).
M. V. Petoukhov and D. I. Svergun, Biophys. J. 89 (2), 1237 (2005).
D. I. Svergun, C. Barberato, and M. H. J. Koch, J. Appl. Crystallogr. 28, 768 (1995).
D. Franke, A. G. Kikhney, and D. I. Svergun, Nucl. Instrum. Methods Phys. Res., Sect. A 689, 52 (2012).
J. Cheng, A. Z. Randall, M. J. Sweredoski, et al., Nucl. Acids Res. 33, 2 (2005).
E. V. Shtykova, L. A. Baratova, N. V. Fedorova, et al., PloS one 8, 1 (2013).
J. Zhang, V. Frey, M. Corcoran, et al., Mol. Pharmaceutics 13, 3362 (2016).
K. Shiraki, M. Kudou, S. Nishikori, et al., Eur. J. Biochem. 271, 3242 (2004).
E. R. Castellanos, C. Ciferri, and W. Phung, Protein Expr. Purif. 124, 10 (2016).
A. J. Dingley, J. R. Mackay, and B. E. Chapman, J. Biomol. NMR 6, 321 (1995).
E. V. Shtykova, Ross. Nanotekhnol. 10, 60 (2015).
X. H. Guo, N. M. Zhao, S. H. Chen, et al., Biopolymers 29, 335 (1990).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Shtykova, E.N. Bogacheva, L.A. Dadinova, C.M. Jeffries, N.V. Fedorova, A.O. Golovko, L.A. Baratova, O.V. Batishchev, 2017, published in Kristallografiya, 2017, Vol. 62, No. 6, pp. 907–916.
Rights and permissions
About this article
Cite this article
Shtykova, E.V., Bogacheva, E.N., Dadinova, L.A. et al. Small-angle X-Ray analysis of macromolecular structure: the structure of protein NS2 (NEP) in solution. Crystallogr. Rep. 62, 894–902 (2017). https://doi.org/10.1134/S1063774517060220
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774517060220