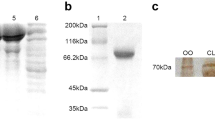
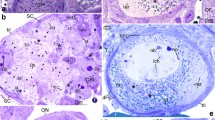
Abstract
During oogenesis, most of the mRNA is localized at the vegetal pole of the oocyte in a special area called the germ plasm. Germ plasm plays an essential and necessary role in the specification of primordial germ cells. We previously characterized the germes gene as an early marker of the germ plasm. In this work, the synthesis of the Germes protein in the ovaries and early development of the clawed frog using the immune serum the authors received was studied. A protein in the follicular cells surrounding the growing oocytes was also found. The fact of the presence of the Germes protein in follicular cells required further investigation into the presence of mRNA in them. For this purpose, method of the in situ hybridization of follicles with a probe against germes combined with electron microscopy were used. Germes mRNA in follicular cells was detected. The transcript was in the cytoplasm and nuclei of the follicular cells themselves, both large and small follicles, and in the space between the oocyte and follicular cells. Comparative analysis of germes mRNA expression in follicular cells and oocytes showed that expression levels in the follicular layer and oocytes of large stages are comparable. The transcript content in the follicular cells of small oocytes exceeded that in the oocytes themselves. It is proven that there is unprocessed mRNA in the follicular layer during the entire oogenesis, which shows the independent expression of the germes germ plasm gene in them.








Similar content being viewed by others
REFERENCES
Berekelya, L.A., Ponomarev, M.B., Luchinskaya, N.N., et al., Xenopus Germes encodes a novel germ plasm-associated transcript, Gene Expr. Patterns, 2003, vol. 3, pp. 521–524.
Berekelya, L.A., Mikryukov, A.A., Luchinskaya, N.N., et al., The protein encoded by the germ plasm RNA Germes associates with dynein light chains and functions in Xenopus germline development, Differentiation, 2007, vol. 75, no. 6, pp. 546–558.
Bilinski, S.M., Jaglarz, M.K., Dougherty, M.T., et al., Electron microscopy, immunostaining, cytoskeleton visualization, in situ hybridization, and three-dimensional reconstruction of Xenopus oocytes, Methods, 2010, vol. 51, no. 1, pp. 11–19.
Bookout, A.L., Cummins, C.L., Mangelsdorf, D.J., et al., High-throughput real-time quantitative reverse transcription PCR, Curr. Protoc. Mol. Biol., 2006, Ch. 15, unt 15.8.
Butler, A.M., Aguero, T., Newman, K.M., et al., Primordial germ cell isolation from Xenopus laevis embryos, Methods Mol. Biol., 2017, vol. 1463, pp. 115–124.
Butler, A.M., Owens, D.A., Wang, L., et al., A novel role for sox7 in Xenopus early primordial germ cell development: mining the PGC transcriptome, Development, 2018, vol. 145, no. 1.
Chan, A.P., Kloc, M., Bilinski, S., et al., The vegetally localized mRNA fatvg is associated with the germ plasm in the early embryo and is later expressed in the fat body, Mech. Dev., 2001, vol. 100, pp. 137–140.
Chang, P., Torres, J., Lewis, R.A., et al., Localization of RNAs to the mitochondrial cloud in Xenopus oocytes through entrapment and association with endoplasmic reticulum, Mol. Biol. Cell, 2004, vol. 15, pp. 4669–4681.
Coggins, L.W., An ultrastructure and radioautographic study of early oogenesis in the toad Xenopus laevis, J. Cell Sci., 1973, vol. 12, pp. 71–93.
Colozza, G. and De Robertis, E.M., Maternal syntabulin is required for dorsal axis formation and is a germ plasm component in Xenopus, Differentiation, 2014, vol. 88, no. 1, pp. 17–26.
Cuykendall, T.N. and Houston, D.W., Identification of germ plasm-associated transcripts by microarray analysis of Xenopus vegetal cortex RNA, Dev. Dyn., 2010, vol. 239, no. 6, pp. 1838–1848.
Houston, D.W., Zhang, J., Maines, J.Z., et al., A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule, Development, 1998, vol. 125, pp. 171–180.
Hudson, C. and Woodland, H.R., Xpat, a gene expressed specifically in germ plasm and primordial germ cells of Xenopus laevis, Mech. Dev., 1998, vol. 73, pp. 190–198.
Ivanov, A.V., Korovina, A.N., Tunitskaya, V.L., et al., Development of the system ensuring a high-level expression of hepatitis C virus nonstructural NS5B and NS5A proteins, Protein Expr. Purif., 2006, vol. 48, no. 1, pp. 14–23.
Konduktorova, V.V. and Luchinskaya, N.N., Follicular cells of the amphibian ovary: origin, structure, and functions, Russ. J. Dev. Biol., 2013, vol. 44, pp. 232–244.
Lai, F., Singh, A., and King, M.L., Xenopus Nanos1 is required to prevent endoderm gene expression and apoptosis in primordial germ cells, Development, 2012, vol. 139, pp. 1476–1486.
Liu, Y., Ren, L., Ge, L., et al., A strategy for fusion expression and preparation of functional glucagon-like peptide-1 (GLP-1) analogue by introducing an enterokinase cleavage site, Biotechnol. Lett., 2014, vol. 36, no. 8, pp. 1675–1680.
MacArthur, H., Houston, D.W. Bubunenko, M., et al., DEADSouth is a germ-plasm-specific DEADbox RNA helicase in Xenopus related to eIF4A, Mech. Dev., 2000, vol. 95, pp. 291–295.
Machado, R.J., Moore, W., Hames, R., et al., Xenopus Xpat protein is a major component of germ plasm and may function in its organisation and positioning, Dev. Biol., 2005, vol. 287, pp. 289–300.
Mosquera, L., Forristall, C., Zhou, Y., et al., A mRNA localized to the vegetal cortex of Xenopus oocytes encodes a protein with a nanos-like zinc finger, Development, 1993, vol. 117, pp. 377–386.
Nandadasa, S., Tao, Q., Menon, N.R., et al., N- and E‑cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements, Development, 2009, vol. 136, no. 8, pp. 1327–1338.
Newport, J. and Kirschner, M., A major developmental transition in early Xenopus embryos. I. Characterisation and timing of cellular changes at the midblastula stage, Cell, 1982, vol. 30, pp. 675–686.
Nieuwkoop, P.D. and Faber, J., Normal Table of Xenopus laevis (Daudin), Amsterdam: North-Holland, 1956.
Ogielska, M. and Kotusz, A., Pattern and rate of ovary differention with reference to somatic development in anuran amphibians, J. Morphol., 2004, vol. 259, pp. 41–54.
Pannese, M., Cagliani, R., Pardini, C.L., et al., Xotx1 maternal transcripts are vegetally localized in Xenopus laevis oocytes, Mech. Dev., 2000, vol. 90, pp. 111–114.
Ponomarev, M.B., Konduktorova, V.V., Luchinskaya, N.N., et al., Localization of Germes RNA in Xenopus oocytes, Russ. J. Dev. Biol., 2021, vol. 52, pp. 1–8.
Rapali, P., García-Mayoral, M.F., Martínez-Moreno, M., et al., LC8 dynein light chain (DYNLL1) binds to the C-terminal domain of ATM-interacting protein (ATMIN/ASCIZ) and regulates its subcellular localization, Biochem. Biophys. Res. Commun., 2011, vol. 414, no. 3, pp. 493–498.
Sambrook, J. and Russell, D., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, 2001.
Tarbashevich, K., Koebernick, K., and Pieler, T., TXGRIP2.1 is encoded by a vegetally localizing, maternal mRNA and functions in germ cell development and anteroposterior PGC positioning in Xenopus laevis, Dev. Biol., 2007, vol. 311, no. 2, pp. 554–565.
Wang, J., Bing, X., Yu, K., et al., Preparation of a polyclonal antibody against goldfish (Carassius auratus) vitellogenin and its application to detect the estrogenic effects of monocrotophos pesticide, Ecotoxicol. Environ. Saf., 2015, vol. 111, pp. 109–116.
Weidinger, G., Stebler, J., Slanchev, K., et al., Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival, Curr. Biol., 2003, vol. 13, no. 16, pp. 1429–1434.
Yamaguchi, T., Taguchi, A., Watanabe, K., et al., DEADsouth protein localizes to germ plasm and is required for the development of primordial germ cells in Xenopus laevis, Biol. Open, 2013, vol. 15, no. 2 (2), pp. 191–199.
Yang, J., Aguero, T., and King, M.L., The Xenopus maternal-to-zygotic transition from the perspective of the germline, Curr. Top. Dev. Biol., 2015, vol. 113, pp. 271–303.
Zearfoss, N.R., Chan, A.P., Wu, C.F., et al., Hermes is a localized factor regulating cleavage of vegetal blastomeres in Xenopus laevis, Dev. Biol., 2004, vol. 267, no. 1, pp. 60–71.
Zhang, J. and King, M.L., Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning, Development, 1996, vol. 122, pp. 4119–4129.
ACKNOWLEDGMENTS
The JEM-1011 microscope with a Gatan ES500W Model 782 camera based on the Moscow State University Collective Use Center was presented with the financial support of the Ministry of Education and Science of the Russian Federation.
We are grateful to Professor H.R. Woodland (Warwick University, United Kingdom), an employee of the Warwick University, United Kingdom, for his methodical help, valuable advice, and consultations.
Funding
The work was carried out within the framework of the state program of research of the Department of Embryology of Moscow State University, no. 30-2-21.
Author information
Authors and Affiliations
Contributions
Victoria Konduktorova performed most of the experiments. All the authors took part in the planning of the experiments. All the authors took part in the discussion of the results. Victoria Konduktorova and Natalia Luchinskaya took part in writing the text of the article.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. In the course of implementing this study, all manipulations with experimental animals and methods of anesthesia complied with international standards on bioethics.
Additional information
Translated by E. Tolkunova
Rights and permissions
About this article
Cite this article
Konduktorova, V.V., Luchinskaya, N.N. & Belyavsky, A.V. Expression of the Germes Germ Plasm Gene in Follicular Cells of X. laevis Oocytes. Russ J Dev Biol 53, 350–362 (2022). https://doi.org/10.1134/S1062360422050034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360422050034