Abstract
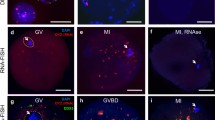
Tandemly repeated pericentromeric noncoding DNA (TR DNA) makes up approximately 10% of the human genome. Pericentromeric TR DNA includes classical human satellites 1, 2, 3 (HS1, HS2, HS3), which are transcribed in somatic cells. We have previously shown the presence of HS2/HS3 transcripts in late human oogenesis and sequenced them. It has been suggested that the RNPs found may be the site of spatial sequestration of RNA and proteins at the end of human oocytes maturation. The aim of this work was to develop a method for inactivating HS2/HS3 transcripts using antisense oligonucleotides to assess its effect on the size and quantity of DDX4-containing RNPs in late human oogenesis. Inactivation of HS2/HS3 transcription at the end of human oocytes maturation by the microinjection method resulted in a significant decrease of the total HS2/HS3 RNA signal, detected by the method of fluorescence in situ hybridization (FISH). At the same time, an increase in the number of inclusions positive to antibodies to RNA helicase DDX4 was observed. Supposedly, upon inactivation of HS2/HS3 transcription, dissociation of DDX4-containing RNP particles occurred. Such changes of the RNP particles can play a critical role in the development of oocytes and be a cause of the arrest of maturation or the occurrence of pathological syndromes, including those associated with problems of fertilization.

Similar content being viewed by others
REFERENCES
Banerjee, P.R., Milin, A.N., Moosa, M.M., Onuchic, P.L., and Deniz, A.A., Reentrant phase transition drives dynamic substructure formation in ribonucleoprotein droplets, Angew. Chem., Int. Ed., 2017, vol. 56, no. 38, pp. 11354–11359. https://doi.org/10.1002/anie.201703191
Blanca, M.J., Alarcón, R., Arnau, J., Bono, R., and Bendayan, R., Non-normal data: is ANOVA still a valid option?, Psicothema, 2017, vol. 29, no. 4, pp. 552–557. https://doi.org/10.7334/PSICOTHEMA2016.383
Ding, Y., Chi, Y.C., and Lawrence, C.E., RNA secondary structure prediction by centroids in a Boltzmann weighted ensemble, RNA, 2005, vol. 11, no. 8, pp. 1157–1166. https://doi.org/10.1261/RNA.2500605
Dobrynin, M.A. and Enukashvily, N.I., Germinal granules in animal oogenesis, Tsitologiya, 2020, vol. 62, no. 12, pp. 851–866.
Dobrynin, M.A., Korchagina, N.M., Prjibelski, A.D., Shafranskaya, D., Ostromyshenskii, D.I., Shunkina, K., Stepanova, I., Kotova, A.V., Podgornaya, O.I., and Enukashvily, N.I., Human pericentromeric tandemly repeated DNA is transcribed at the end of oocyte maturation and is associated with membraneless mitochondria-associated structures, Sci. Rep., 2020, vol. 10, no. 1, pp. 1–15. https://doi.org/10.1038/s41598-020-76628-8
Enukashvily, N.I. and Ponomartsev, N.V., Mammalian satellite DNA: a speaking dumb, Adv. Protein Chem. Struct. Biol., 2013, vol. 90, pp. 31–65.https://doi.org/10.1016/B978-0-12-410523-2.00002-X
Enukashvily, N.I., Dobrynin, M.A., and Chubar, A.V., Rna-seeded membraneless bodies: role of tandemly repeated RNA, Adv. Protein Chem. Struct. Biol., Academic, 2021, vol. 126, pp. 151–193. ISSN 1876-1623. https://doi.org/10.1016/bs.apcsb.2020.12.007
Hubstenberger, A., Noble, S.L., Cameron, C., and Evans, T.C., Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development, Dev. Cell, 2013, vol. 27, no. 2, pp. 161–173. https://doi.org/10.1016/j.devcel.2013.09.024
Jain, A. and Vale, R.D., RNA phase transitions in repeat expansion disorders, Nature, 2017, vol. 546, no. 7657, pp. 243–247. https://doi.org/10.1038/nature22386
Lennox, K.A. and Behlke, M.A., Chemical modification and design of anti-miRNA oligonucleotides, Gene Ther., 2011, vol. 18, no. 12, pp. 1111–1120. https://doi.org/10.1038/GT.2011.100
Maharana, S., Wang, J., Papadopoulos, D.K., Richter, D., Pozniakovsky, A., Poser, I., Bickle, M., Rizk, S., Guillen-Boixet, J., Franzmann, T.M., Jahnel, M., Marrone, L., Chang, Y.T., Sterneckert, J., Tomancak, P., Hyman, A.A., and Alberti, S., RNA buffers the phase separation behavior of prion-like RNA binding proteins, Science, 2018, vol. 360, no. 6391, pp. 918–921. https://doi.org/10.1126/SCIENCE.AAR7366
Namkoong, S., Ho, A., Woo, Y.M., Kwak, H., and Lee, J.H., Systematic characterization of stress-induced RNA granulation, Mol. Cell, 2018, vol. 70, no. 1, pp. 175–187. e8. https://doi.org/10.1016/J.MOLCEL.2018.02.025
Rangan, P., DeGennaro, M., Jaime-Bustamante, K., Coux, R.-X., Martinho, R., and Lehmann, R., Temporal and spatial control of germ-plasm RNAs, Curr. Biol., 2009, vol. 19, no. 1, pp. 72–77. https://doi.org/10.1016/J.CUB.2008.11.066
Reunov, A.A. and Reunova, Y.A., In mouse oocytes the mitochondrion-originated germinal body-like structures accumulate mouse Vasa homologue (MVH) protein, Zygote, 2015, vol. 23, no. 4. https://doi.org/10.1017/S0967199414000124
Rhine, K., Vidaurre, V., and Myong, S., RNA droplets, Annu. Rev. Biophys., 2020, vol. 49, p. 247. https://doi.org/10.1146/ANNUREV-BIOPHYS-052118-115508
Richard, G.-F., Kerrest, A., and Dujon, B., Comparative genomics and molecular dynamics of DNA repeats in eukaryotes, Microbiol., Mol. Biol. Rev., 2008, vol. 72, no. 4, pp. 686–727. https://doi.org/10.1128/MMBR.00011-08
De Smedt, V., Szöllösi, D., and Kloc, M., The Balbiani body: asymmetry in the mammalian oocyte, Genesis, 2000, vol. 26, no. 3, p. 208. https://doi.org/10.1002/(sici)1526-968x(200003)26:3<208::aid-gene6>3.3.co;2-e
Trofimova, I.L., Enukashvily, N.I., Kuznetsova, T.V., and Baranov, V.S., Transcription of satellite DNA in human embryogenesis: literature review and own data, Med. Genet., 2018, vol. 17, no. 3, pp. 3–7.
Valgardsdottir, R., Chiodi, I., Giordano, M., Cobianchi, F., Riva, S., and Biamonti, G., Structural and functional characterization of noncoding repetitive RNAs transcribed in stressed human cells, Mol. Biol. Cell, 2005, vol. 16, no. 6, pp. 2597–2604. https://doi.org/10.1091/MBC.E04-12-1078/ASSET/IMAGES/LARGE/ZMK0060571650005.JPEG
Yandlm, C. and Karakülah, G., Expression dynamics of repetitive DNA in early human embryonic development, BMC Genomics, 2019, vol. 20. https://doi.org/10.1186/S12864-019-5803-1
Funding
The study was carried out with the financial support of a grant from the Ministry of Science and Higher Education of the Russian Federation, no. 075-15-2021-1075 dated September 28, 2021 and grant of the Russian Science Foundation, no. 19-74-20102 (collection of oocytes, design of antisense primers).
Author information
Authors and Affiliations
Contributions
Development of the research concept, planning of experiments: N.I. Enukashvily, N.M. Korchagina, M.A. Dobrynin; conducting experiments: M.A. Dobrynin, N.V. Korchagina; methodology: N.M. Korchagina, N.V. Ponomartsev, N.I. Enukashvily; visualization: M.A. Dobrynin; financial support: O.I. Podgornaya, N.I. Enukashvily; manuscript preparation: M.A. Dobrynin, N.I. Enukashvily.
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
All oocytes were received from donors according to the order no. 803n the Ministry of Health of the Russian Federation on July 31, 2021, and in line with the Word Medical Association (WMA) Helsinki Declaration (Declaration of Helsinki: Ethical Principles for Medical Research Involving People, including the amendments to the 64th meeting of the SCA in Fortaleza, Brazil, October 2013). The study was approved by the local Ethics Committee of the Ava-Peter-Scandinavia clinics (no. 11/22-12-2016). Written informed consent was obtained from each donor included in the study.
Additional information
The data were presented at the conference of young scientists “Actual Problems of Developmental Biology” on October 12–14, 2021, Moscow, Koltzov Institute of Developmental Biology, Russian Academy of Sciences
Translated by E.N. Tolkunova
Rights and permissions
About this article
Cite this article
Dobrynin, M.A., Korchagina, N.M., Ponomartsev, N.V. et al. Influence of Inactivation of Tandemly Repeated Pericentromeric DNA Transcription on the Formation of Membraneless Structures at the End of Oocyte Maturation. Russ J Dev Biol 53, 128–133 (2022). https://doi.org/10.1134/S1062360422020059
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360422020059