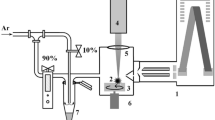
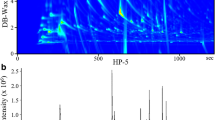
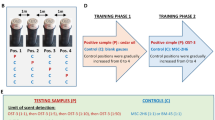
Abstract—
The effect of the stage of development of a malignant tumor on successful detection in the urine of animals with oncology by dogs was studied. It was demonstrated that the success of dogs in the search for sick organisms among healthy ones is quite consistent with the dynamics of the development of transplanted tumor tissue; that is, dogs are able to perceive both the entire spectrum of volatile metabolites accompanying the disease and changes in this spectrum associated directly with the development of the tumor. The existence of volatile organic compounds associated with the growth of a malignant tumor was established.



Similar content being viewed by others
REFERENCES
Amal, H., Ding, L., Liu, B.B., Tisch, U., Xu, Z.Q., Shi, D.Y., Zhao, Y., Chen, J., Sun, R.X., Liu, H., Ye, S.L., Tang, Z.Y., and Haick, H., The scent fingerprint of hepatocarcinoma: in-vitro metastasis prediction with volatile organic compounds (VOCs), Int. J. Nanomed., 2012, vol. 7, pp. 4135–4146.
Amann, A., Breath analysis for clinical diagnosis and therapeutic monitoring, Siriraj Med. J., 2012, vol. 6, suppl. 2, pp. 18–19.
American Lung Association. Lung cancer fact sheet. American Lung Association, 2015. http://www. lung. org/lung-health-and-diseases/lung-disease-lookup/lung-cancer/resource-library/lung-cancer-fact-sheet.html. Accessed March 22, 2020.
Arakawa, H., Arakawa, K., and Deak, T., Sickness-related odor communication signals as determinants of social behavior in rat: a role for inflammatory processes, Horm. Behav., 2010, vol. 57, no. 3, pp. 330–341.
Arakawa, H., Cruz, S., and Deak, T., From models to mechanisms: odorant communication as a key determinant of social behavior in rodents during illness-associated states, Neurosci. Biobehav. Rev., 2011, vol. 35, no. 9, pp. 1916–1928.
Beauchamp, G.K. and Yamazaki, K., Individual differences and the chemical senses, Chem. Senses, 2005, vol. 30, suppl. 1, pp. i6–i9.
Beauchamp, G.K., Yamazaki, K., Wysocki, C.J., Slotnick, B.M., Thomas, L., and Boyse, E.A., Chemosensory recognition of mouse major histocompatibility types by another species, Proc. Natl. Acad. Sci. U. S. A., 1985, vol. 82, pp. 4186–4188.
Bekoff, M., Observations of scent-marking and discriminating self from others by a domestic dog (Canis familiaris): tales of displaced yellow snow, Behav. Process, 2001, vol. 55, no. 2, pp. 75–79.
Bijland, L.R., Bomers, M.K., and Smulders, Y.M., Smelling the diagnosis. A review on the use of scent in diagnosing, Neth. J. Med., 2013, vol. 71, pp. 300–307.
Boots, A.W., van Berkel, J.J.B.N., Dallinga, J.W., Smolinska, A., Wouters, M.F.M., and van Schooten, F.J., The versatile use of exhaled volatile organic compounds in health and disease, J. Breath Res., 2012, vol. 6, p. 027108.
Brennan, P.A. and Kendrick, K.M., Mammalian social odours: attraction and individual recognition, Philos. Trans. R. Soc. Lond., B: Biol. Sci., 2006, vol. 361, pp. 2061–2078.
Chambers, S.T., Scott-Thomas, A., and Epton, M., Developments in novel breath tests for bacterial and fungal pulmonary infection, Curr. Opin. Pulm. Med., 2012, vol. 18, pp. 228–232.
Cicolella, A., Volatile organic compounds (VOC): definition, classification and properties, Rev. Mal. Respir., 2008, vol. 25, pp. 155–163.
Cornu, J.N., Cancal-Tassin, G., Ondet, V., Girardet, C., and Cussenot, O., Olfactory detection of prostate cancer by dogs sniffing urine: a step forward to early diagnosis, Eur. Urol., 2011, vol. 59, pp. 197–201.
Dummer, J., Storer, SwanneyM., McEwan, M., Scott-Thomsad, A., Bhandari, S., Chambers, S., Dweik, R., and Epton, M., Analysis of biogenic volatile organic compounds in health and disease, TrAC, Trends Anal. Chem. (Pers. Ed.), 2011, vol. 30, pp. 960–967.
Ehman, K.D. and Scott, M.E., Female mice mate preferentially with non-parasitized males, Parasitology, 2002, vol. 125, no. 5, pp. 461–466.
Ehmann, R., Boedeker, E., Friedrich, U., Sagert, J., Dippon, J., Friedel, G., and Walles, T., Canine scent detection in the diagnosis of lung cancer: revisiting a puzzling phenomenon, Eur. Resp. J., 2011, vol. 39, pp. 669–676.
El-Serag, H.B., Marrero, J.A., Rudolph, L., and Reddy, K.R., Diagnosis and treatment of hepatocellular carcinoma, Gastroenterology, 2008, vol. 134, pp. 1752–1763.
Filipiak, W., Sponring, A., Mikoviny, T., Ager, C., Schubert, J., Miekisch, W., Amann, A., and Troppmair, J., Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU-1 in vitro, Cancer Cell Int., 2008, vol. 8, no. 1, pp. 17–28.
Fouad, Y.A. and Aanei, C., Revisiting the hallmarks of cancer, Am. J. Cancer Res., 2017, vol. 7, no. 5, pp. 1016–1036.
Garner, C.E., Smith, S., Costello, B., White, P., Spencer, R., Prober, C.S.J., and Ratcliffe, M., Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease, FASEB J., 2007, vol. 21, no. 8, pp. 1675–1688.
Garner, C.E., Smith, S., Bardhan, P.K., Ratcliffe, N.M., and Probert, C.S., A pilot study of faecal volatile organic compounds in faeces from cholera patients in Bangladesh to determine their utility in disease diagnosis, Trans. R. Soc. Trop. Med. Hyg., 2009, vol. 103, no. 11, pp. 1171–1173.
Guernion, N., Ratcliffe, M., Spencer-Phillips, P.T., and Howe, R.A., Identifying bacteria in human urine: current practice and the potential for rapid, near-patient diagnosis by sensing volatile organic compounds, Clin. Chem. Lab. Med., 2001, vol. 39, pp. 893–906.
Hakim, M., Broza, Y.Y., Barash, O., Peled, N., Phillips, M., Amann, A., and Haick, H., Volatile organic compounds of lung cancer and possible biochemical pathways, Chem. Rev., 2012, vol. 112, pp. 5949–5966.
Hanahan, D. and Weinberg, R.A., Hallmarks of cancer: the next generation, Cell, 2011, vol. 144, no. 5, pp. 646–674.
Harrington, F.H., Asa, C.S., Mech, L., and Boitani, L., Wolf communication, in Wolves: Behavior, Ecology, and Conservation, Chicago: Univ. Chicago Press, 2003, vol. 3, pp. 66–103.
Hartke, J., Johnson, M., and Ghabril, M., The diagnosis and treatment of hepatocellular carcinoma, Semin. Diagn. Pathol., 2017, vol. 34, pp. 153–159.
Horvath, I., Lazar, Z., Gyulai, N., Kollai, M., and Losonczy, G., Exhaled biomarkers in lung cancer, Eur. Respir. J., 2009, vol. 34, pp. 261–275.
Jett, J.R., Limitations of screening for lung cancer with low-dose spiral computed tomography, Clin. Cancer Res., 2005, vol. 11, no. 13, pp. 4988s–4992s.
Johnston, R.E., Chemical communication in rodents: from pheromones to individual recognition, J. Mammal., 2003, vol. 84, pp. 1141–1162.
Kang, J., Zhu, L., Lu, J., and Zhang, X., Application of metabolomics in autoimmune diseases: insight into biomarkers and pathology, J. Neuroimmunol., 2015, vol. 279, pp. 25–32.
Kavaliers, M., Choleris, E., and Pfaff, D.W., Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates, Neurosci. Biobehav. Rev., 2005a, vol. 29, pp. 1347–1359.
Kavaliers, M., Choleris, E., Agmo, A., Muglia, L.J., Ogawa, S., and Pfaff, D.W., Involvement of the oxytocin gene in the recognition and avoidance of parasitized males by female mice, Anim. Behav., 2005b, vol. 70, pp. 693–702.
Keller, M., Douhard, Q., Baum, M.J., and Bakker, J., Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice, Chem. Senses, 2006a, vol. 31, pp. 315–323.
Keller, M., Pierman, S., Douhard, Q., Baum, M.J., and Bakker, J., The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice, Eur. J. Neurosci., 2006b, vol. 23, pp. 521–530.
Kelliher, K.R., The combined role of the main olfactory and vomeronasal systems in social communication in mammals, Horm. Behav., 2007, vol. 52, pp. 561–570.
Kimball, B.A., Volatile metabolome: problems and prospects, Bioanalysis, 2016a, vol. 8, no. 19, pp. 1987–1991.
Kimball, B.A., Yamazaki, K., Kohler, D., Bowen, R.A., Muth, J.P., Opiekun, M., and Beauchamp, G.K., Avian influenza infection alters fecal odor in mallards, PLoS One, 2013, vol. 8, no. 10. e75411.
Kimball, B.A., Opiekun, M., Yamazaki, K., and Beauchamp, G.K., Immunization alters body odor, Physiol. Behav., 2014, vol. 128, pp. 80–85.
Kimball, B.A., Wilson, D.A., and Wesson, D.W., Alterations of the volatile metabolome in mouse models of Alzheimer’s disease, Sci. Rep., 2016b, vol. 14, no. 6, p. 19495.
Kimball, B.A., Cohen, A.S., Gordon, A.R., Opiekun, M., Martin, T., Elkind, J., Lundstrom, J.N., and Beauchamp, G.K., Brain injury alters volatile metabolome, Chem. Senses, 2016c, vol. 41, no. 5, pp. 407–414.
Kochevalina, M.Yu. and Trunov, V.G., Principles of organizing a database for behavioral experiments with macrosmatic animals, Usp. Sovrem. Nauki, 2016, vol. 3, no. 4, pp. 130–133.
Kochevalina, M.Yu., Rodionova, E.I., Morozova, O.V., Ambaryan, A.V., and Borodkov, A.S., The study of the odorological profile of the urine of mice with hepatocarcinoma, Tr. Conf. IT, 2014, pp. 570–574. http://itas2014.iitp.ru/pdf/1569927987.pdf.
Krutova, V.I., Sulimov, K.T., and Zinkevich, E.P., The time of appearance of an individual odor in the ontogenesis of gray rats (Rattus norvegicus) according to cynological analysis, Sensorn.Sist., 1997, vol. 11, no. 3, pp. 340–345.
Lazarevich, N.L., Cheremnova, O.A., Varga, E.V., Ovchinnikov, D.A., Kudrjavtseva, E.I., Morozova, O.V., Fleishman, D.I., Engelhardt, N.V., and Duncan, S.A., Progression of HCC in mice is associated with a downregulation in the expression of hepatocyte nuclear factors, Hepatology, 2004, vol. 39, pp. 1038–1047.
Liddell, K., Smell as a diagnostic marker, Postgrad. Med. J., 1976, vol. 52, no. 605, pp. 136–138.
Lubes, G. and Goodarzi, M., GC-MS based metabolomics used for the identification of cancer volatile organic compounds biomarkers, J. Pharm. Biomed. Anal., 2017, vol. 147, pp. 313–322.
Ludwig, J.A. and Weinstein, J.N., Biomarkers in cancer staging, prognosis and treatment selection, Nat. Rev. Cancer, 2005, vol. 5, no. 11, pp. 845–856.
Masuda, T. and Miyoshi, E., Cancer biomarkers for hepatocellular carcinomas: from traditional markers to recent topics, Clin. Chem. Lab. Med., 2011, vol. 49, no. 6, pp. 959–966.
Matsumura, K., Opiekun, M., Oka, H., Vachani, A., Albelda, S.M., Yamazaki, K., and Beauchamp, G.K., Urinary volatile compounds as biomarkers for lung cancer: a proof of principle study using odor signatures in mouse models of lung cancer, PLoS One, 2010, vol. 5, no. 1. e8819.
Mazzone, P.J., Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer, J. Thorac. Oncol., 2008, vol. 3, pp. 774–780.
Miekisch, W., Schubert, J.K., Gabriele, F.E., and Noelge-Schomburg, G.F.E., Diagnostic potential of breath analysis—focus on volatile organic compounds, Clin. Chim. Acta, 2004, vol. 347, pp. 25–39.
Minino, A.M. and Kochanek, K.D., Deaths: preliminary data for 2008, Natl. Vital Stat. Rep., 2010, vol. 59, pp. 1–72.
Moser, E. and McCulloch, M., Canine scent detection of human cancers: a review of methods and accuracy, J. Vet. Behav. Clin. Appl. Res., 2010, vol. 5, pp. 145–152.
Novak, B.J., Blake, D.R., Meinardi, S., Rowland, F.S., Pontello, A., Cooper, D.M., and Galassetti, P.R., Exhaled methyl nitrate as a noninvasive marker of hyperglycemia in type 1 diabetes, Proc. Natl. Acad. Sci. U. S. A., 2007, vol. 104, pp. 15613–15618.
Novotny, M., Harvey, S., Jemiolo, B., and Alberts, J., Synthetic pheromones that promote inter-male aggression in mice. Proc. Natl. Acad. Sci. U. S. A., 1985, vol. 82, pp. 2059–2061.
Osada, K., Yamazaki, K., Curran, M., Bard, J., Smith, B.P., and Beauchamp, G.K., The scent of age, Proc. R. Soc. Lond. B. Biol. Sci., 2003, vol. 270, no. 1518, pp. 929–933.
Osada, K., Tashiro, T., Mori, K., and Izumi, H., The identification of attractive volatiles in aged male mouse urine, Chem. Senses, 2008, vol. 33, no. 9, pp. 815–823.
Parkin, D.M., Bray, F., Ferlay, J., and Pisani, P., Estimating the world cancer burden: globocan 2000, Int. J. Cancer, 2001, vol. 94, no. 2, pp. 153–156.
Pavlou, A.K. and Turner, A.P., Sniffing out the truth: clinical diagnosis using the electronic nose, Clin. Chem. Lab. Med., 2000, vol. 38, pp. 99–112.
Peng, G., Hakim, M., Broza, Y.Y., Billan, S., Abdah-Bortnyak, R., Kuten, A., Tisch, U., and Haick, H., Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors, Br. J. Cancer, 2010, vol. 103, pp. 542–551.
Penn, D. and Potts, W.K., Chemical signals and parasite-mediated sexual selection, Trends Ecol. Evol., 1998, vol. 13, pp. 391–396.
Phillips, M., Cataneo, R.N., Cheema, T., and Greenberg, J., Increased breath biomarkers of oxidative stress in diabetes mellitus, Clin. Chim. Acta, 2004, vol. 344, pp. 189–194.
Phillips, M., Cataneo, R.N., Condos, R. Ring, EriksonG.A., and Greenberg, J., La Bombardi, V., Munawar, M.I., and Tietje, O., Volatile biomarkers of pulmonary tuberculosis in the breath, Tuberculosis, 2007, vol. 87, pp. 44–52.
Probert, C.S., Ahmed, I., Khalid, T., Johnson, E., Smith, S., and Ratcliffe, N., Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases, J. Gastrointestin. Liver Dis., 2009, vol. 18, no. 3, pp. 337–343.
Qin, T., Liu, H., Song, Q., Song, G., Wang, H.Z., Pan, Y.Y., Xiong, F.X., Gu, K.S., Sun, G.P., and Chen, Z.D., The screening of volatile markers for hepatocellular carcinoma, Cancer Epidemiol. Biomark. Prev., 2010, vol. 19, no. 9, pp. 2247–2253.
Rodionova, E.I., Kochevalina M.Yu., Kotenkova E.V., Morozova O.V., Kogun’ G.A., Bataeva, E.L., and Ambaryan, A.V., Detection of volatile organic compounds associated with hepatocellular carcinoma by macrosmatic animals: approaches to the search for new tumor markers, Biol. Bull. (Moscow), 2015, vol. 42, no. 3, pp. 239–245.
Rozhok, A.I. and DeGregori, J., The evolution of lifespan and age-dependent cancer risk, Trends Cancer, 2016, vol. 2, no. 10, pp. 552–560.
Sato, T., Katsuoka, Y., Yoneda, K., Nonomura, M., Uchimoto, S., Kobayakawa, R., Kobayakawa, K., and Mizutani, Y., Sniffer mice discriminate urine odours of patients with bladder cancer: a proof-of-principle study for non-invasive diagnosis of cancer-induced odour, Sci. Rep., 2017, vol. 7, no. 1, p. 14628.
Schaal, B., Coureaud, G., Langlois, D., Ginies, C., Semon, E., and Perrier, G., Chemical and behavioural characterization of the rabbit mammary pheromone, Nature, 2003, vol. 424, pp. 68–72.
Schaefer, M.L., Yamazaki, K., Osada, K., Restrepo, D., and Beauchamp, G.K., Olfactory fingerprints for major histocompatibility complex-determined body odors ii: relationship among odor maps, genetics, odor composition, and behavior, J. Neurosci., 2002, vol. 22, pp. 9513–9521.
Schellinck, H.M., West, A.M., and Brown, R.E., Rats can discriminate between the urine odors of genetically identical mice maintained on different diets, Physiol. Behav., 1992, vol. 51, no. 5, pp. 1079–1082.
Schmidt, K. and Podmore, I., Current challenges in volatile organic compounds analysis as potential biomarkers of cancer, J. Biomark., 2015, vol. 2015, art. ID 981458.
Schoon, G.A.A., Scent identification lineups by dogs (Canis familiaris): experimental design and forensic application, Appl. Anim. Behav. Sci., 1996, vol. 49, pp. 257–267.
Shirasu, M. and Touhara, K., The scent of disease: volatile organic compounds of the human body related to disease and disorders, J. Biochem., 2011, vol. 150, pp. 257–266.
Strauch, M., Lüdke, A., Münch, D., Laudes, T., Galizia, C.G., Martinelli, E., Lavra, L., Paolesse, R., Ulivieri, A., Catini, A., and Capuano, R., More than apples and oranges—detecting cancer with a fruit fly’s antenna, Sci. Rep., 2014, vol. 4, p. 3576.
Syhre, M., Manning, L., Phuanukoonnon, S., Harino, P., and Chambers, S.T., The scent of mycobacterium tuberculosis—part II breath, Tuberculosis, 2009, vol. 89, no. 4, pp. 263–266.
Trinchet, J.C., Alperovitch, A., Bedossa, P., Degos, F., Hainaut, P., and Beers, B.V., Epidemiology, prevention, screening and diagnosis of hepatocellular carcinoma, Bull. Cancer, 2009, vol. 96, pp. 35–43.
Wells, M.C. and Bekoff, M., An observational study of scent-marking in coyotes, Canis latrans,Anim. Behav., 1981, vol. 29, no. 2, pp. 332–350.
Willis, C. and Poulin, R., Preference of female rats for the odours of non-parasitised males: the smell of good genes?, Folia Parasitol., 2000, vol. 47, no. 1, pp. 6–10.
Yamazaki, K., Beauchamp, G.K., Singer, A., Bard, J., and Boyse, E.A., Odor types: their origin and composition, Proc. Natl. Acad. Sci. U. S. A., 1999, vol. 96, pp. 1522–1525.
Yamazaki, K., Boyse, E.A., Bard, J., Curran, M., Kim, D., Ross, S.R., and Beauchamp, G.K., Presence of mouse mammary tumor virus specifically alters the body odor of mice, Proc. Natl. Acad. Sci. U. S. A., 2002, vol. 99, no. 8, pp. 5612–5615.
Zlotta, A.R. and Nam, R.K., To biopsy or not to biopsy— thou shall think twice, Eur. Urol., 2012, vol. 61, no. 6, pp. 1115–1117.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Kochevalina, M.Y., Trunov, V.G., Morozova, O.V. et al. Change in Urine Odor of Mice in the Dynamics of Formation of a Transplanted Hepatocarcinoma H33 Tumor. Biol Bull Russ Acad Sci 47, 506–513 (2020). https://doi.org/10.1134/S1062359020050052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359020050052